The limited natural capacity for articular cartilage to regenerate has led to a continuously broadening array of surgical interventions. Used once patients’ symptoms are not relieved by nonoperative management, these share the goals of joint preservation and restoration. Techniques include bone marrow stimulation, whole-tissue transplantation, and cell-based strategies, each with its own variations. Many of these interventions are performed arthroscopically or with extended-portal techniques. Indications, operative techniques, unique benefits, and limitations are presented.
Key points
- •
Articular cartilage has limited intrinsic healing ability. The goal of surgical intervention is joint preservation through cartilage repair or restoration.
- •
Bone marrow stimulation, whole-tissue transplantation, and cell-based strategies are broad treatment concepts for these injuries. Technique variations exist within each division.
- •
Substantial differences often exist among interventions regarding the size of injury that can be addressed, anatomic location, technical considerations, cost, and expectations. These differences warrant transparent discussion with patients about specific goals of treatment and require at least a basic understanding of available surgical options.
Introduction
Articular surface injuries present a common and challenging problem for the musculoskeletal physician. This difficulty is secondary to the complex structure of articular cartilage and its limited natural capacity for regeneration. Furthermore, these injuries frequently occur in a younger patient population with potential for significant effects on quality of life. Many nonoperative treatment modalities are widely used and typically considered first-line management, though this depends on the size, location, and other injury characteristics. For persistently symptomatic or larger articular injuries, surgical intervention often can provide substantial improvement in symptoms and functional capacity.
Surgical options for chondral and osteochondral injury range from procedures traditionally considered primary or reparative to traditionally secondary or restorative. These procedures include open reduction and internal fixation, bone marrow stimulation, whole-tissue transplantation, and cell-based strategies. The purpose of these techniques is surface reconstitution, with the ideal goal of mature, organized, hyaline cartilage. Some interventions achieve this better than others.
Many of these techniques are performed arthroscopically or with arthroscopic assistance. Vast research has been reported on this broad and evolving field, much of which is from the knee literature. Earlier interventions have naturally received more attention and rigorous study than more recent ones. Many newer developments remain in the early stages of determining indications and long-term outcomes, some with little support for efficacy. Accordingly, reviews of more recently developed interventions are more superficial, though with anticipation of what their potential may hold.
Evaluation of outcomes has been largely through comparative studies of various surgical methods, as the natural history of articular lesions remains poorly defined. In addition, a paucity of randomized controlled trials has made robust comparisons between techniques statistically challenging, and results must be interpreted in this context.
With an increasingly large area of injury, arthritic severity, or advancing age, definitive management often is arthroplasty or arthrodesis. There are exceptions to this with the capabilities of bulk osteochondral allografting. As arthroplasty and arthrodesis are considered neither reparative nor restorative, their role is not included in this review.
Of note, considerations of periarticular biomechanical factors, such as malalignment adjacent to the injured joint and soft-tissue deficiencies (meniscus, labrum, and so forth) are extremely important. Although outside the scope of this article, these can strongly influence whether joint salvage will be successful or predicted to fail and must always receive careful consideration in surgical decision-making. These procedures may be performed in a staged or concomitant fashion. Independent of whether these complicating factors are present, transparent discussion between the physician and patient regarding goals is required. This discussion allows review of evidence-based outcomes and provides insight into specific expectations of this typically younger, physically active population.
Introduction
Articular surface injuries present a common and challenging problem for the musculoskeletal physician. This difficulty is secondary to the complex structure of articular cartilage and its limited natural capacity for regeneration. Furthermore, these injuries frequently occur in a younger patient population with potential for significant effects on quality of life. Many nonoperative treatment modalities are widely used and typically considered first-line management, though this depends on the size, location, and other injury characteristics. For persistently symptomatic or larger articular injuries, surgical intervention often can provide substantial improvement in symptoms and functional capacity.
Surgical options for chondral and osteochondral injury range from procedures traditionally considered primary or reparative to traditionally secondary or restorative. These procedures include open reduction and internal fixation, bone marrow stimulation, whole-tissue transplantation, and cell-based strategies. The purpose of these techniques is surface reconstitution, with the ideal goal of mature, organized, hyaline cartilage. Some interventions achieve this better than others.
Many of these techniques are performed arthroscopically or with arthroscopic assistance. Vast research has been reported on this broad and evolving field, much of which is from the knee literature. Earlier interventions have naturally received more attention and rigorous study than more recent ones. Many newer developments remain in the early stages of determining indications and long-term outcomes, some with little support for efficacy. Accordingly, reviews of more recently developed interventions are more superficial, though with anticipation of what their potential may hold.
Evaluation of outcomes has been largely through comparative studies of various surgical methods, as the natural history of articular lesions remains poorly defined. In addition, a paucity of randomized controlled trials has made robust comparisons between techniques statistically challenging, and results must be interpreted in this context.
With an increasingly large area of injury, arthritic severity, or advancing age, definitive management often is arthroplasty or arthrodesis. There are exceptions to this with the capabilities of bulk osteochondral allografting. As arthroplasty and arthrodesis are considered neither reparative nor restorative, their role is not included in this review.
Of note, considerations of periarticular biomechanical factors, such as malalignment adjacent to the injured joint and soft-tissue deficiencies (meniscus, labrum, and so forth) are extremely important. Although outside the scope of this article, these can strongly influence whether joint salvage will be successful or predicted to fail and must always receive careful consideration in surgical decision-making. These procedures may be performed in a staged or concomitant fashion. Independent of whether these complicating factors are present, transparent discussion between the physician and patient regarding goals is required. This discussion allows review of evidence-based outcomes and provides insight into specific expectations of this typically younger, physically active population.
Indications
Considering the several joints most commonly affected by chondral injuries and their differing anatomic structure, function, and weight-bearing demands, this injury group represents a heterogeneous population. However, broad, generalized indications for chondral and osteochondral interventions may be inferred from this vast body of investigation. These indications are largely extrapolated from knee literature, though frequently applied to other joints, serving as their framework. Most investigators agree that indications include patient age ranging from skeletal maturity (depending on the procedure) to 40 to 50 years, well-preserved adjacent cartilage surfaces with minimal or no surrounding signs of osteoarthritis, noninflammatory arthritis, focal full-thickness cartilage defects (Modified Outerbridge or International Cartilage Repair Society [ICRS] grade 3 or 4), and patient ability and willingness to participate in a rigorous postoperative physical therapy regimen ( Table 1 ). Defect depth and area guide the decision on surgical technique. A joint-specific example of such considerations for the knee is shown in Table 2 . More than one defect may be surgically treated, though outcomes have not been as successful if the lesions are “bipolar” or “kissing (present in the same area, on opposing surfaces of the joint).”
| Grade | Outerbridge | Modified Outerbridge | ICRS |
|---|---|---|---|
| 0 | Normal cartilage | Intact cartilage | Intact cartilage |
| I | Softening and swelling | Chondral softening or blistering with intact surface | Superficial (soft indentation or superficial fissures and cracks) |
| II | Fragmentation and fissures in area <0.5-in diameter | Superficial ulceration, fibrillation, or fissuring <50% of depth of cartilage | Lesion less than half the thickness of articular cartilage |
| III | Fragmentation and fissures in area >0.5-in diameter | Deep ulceration, fibrillation, fissuring, or chondral flap >50% of cartilage without exposed bone | Lesion more than half the thickness of articular cartilage |
| IV | Exposed subchondral bone | Full-thickness wear with exposed subchondral bone | Lesion extending to subchondral bone |
| Lesion Size | Operative Treatment |
|---|---|
| ≤1.0 cm | Observation Abrasion chondroplasty Microfracture Osteochondral autograft transfer |
| 1.0–2.0 cm | Abrasion chondroplasty Microfracture Osteochondral autograft transfer |
| 2.0–3.5 cm | Fresh osteochondral allograft Autologous chondrocyte implantation |
| 3.5–10.0 cm | Autologous chondrocyte implantation |
| Multiple (2 or 3) | Autologous chondrocyte implantation |
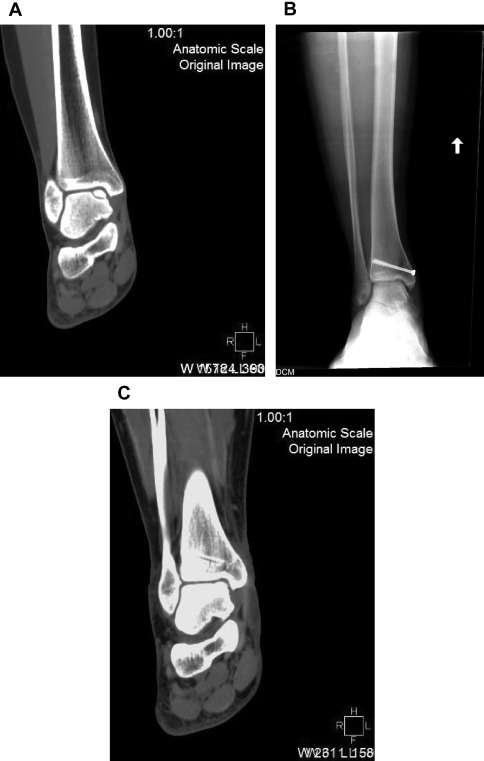
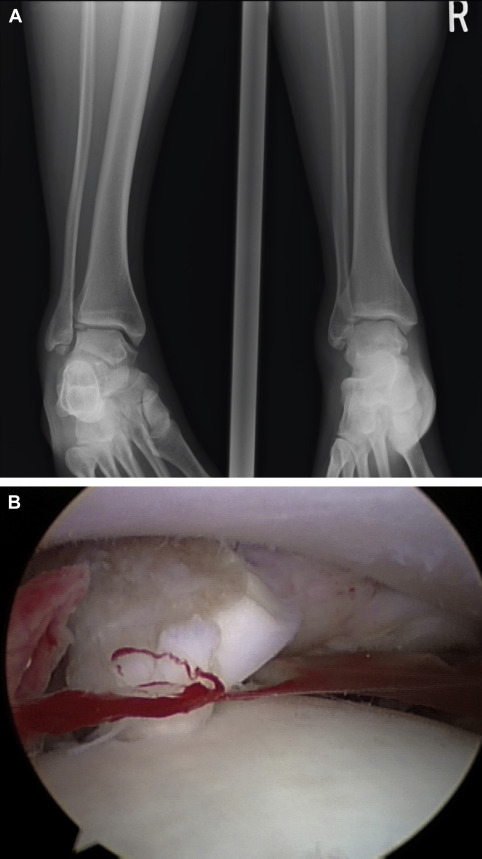
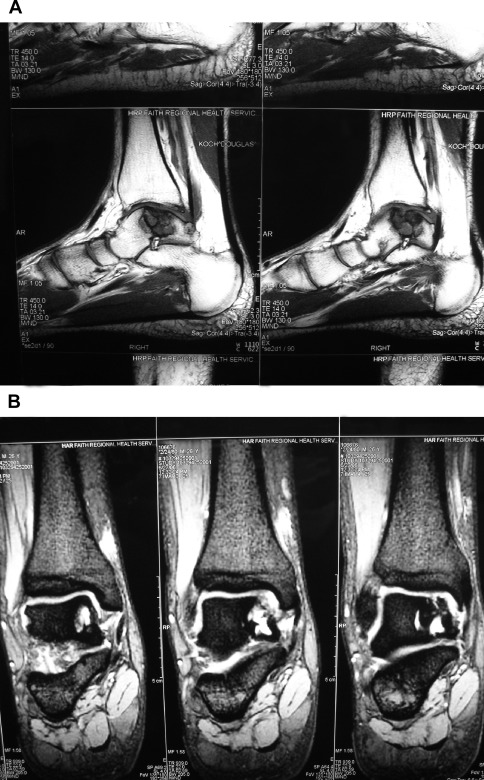
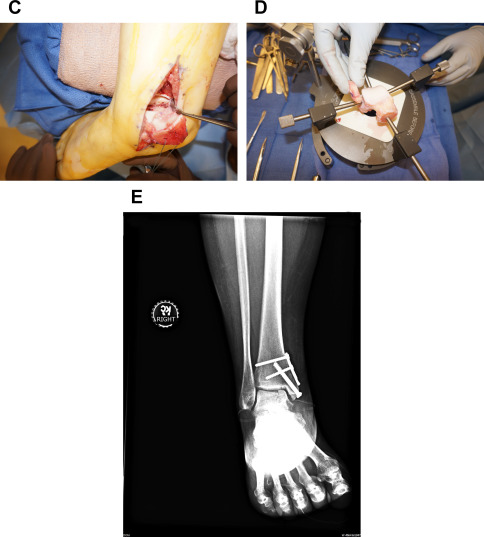
An important consideration must always be the potential for direct repair of a displaced fragment with open reduction and internal fixation (ORIF). Preoperative imaging will determine if this may be possible. If so, this should always be attempted so as to preserve patients’ native articular surface ( Fig. 1 ). The described lateral inverted osteochondral fracture of the talus (LIFT) lesion is an example of an injury that may be suitable for this technique ( Fig. 2 ). The surgeon must ensure that required implants are available at the time of surgery, both for ORIF and an alternative technique if needed. The need for an operative backup plan often accompanies these injuries. The following surgical interventions are indicated when direct reduction and fixation are not possible or have been attempted and failed.
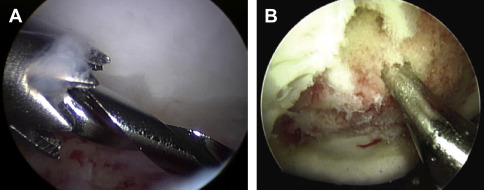
Microfracture
Bone marrow stimulation, most commonly with a microfracture (MF) technique, is the most frequently performed first-line surgical management of a small full-thickness chondral defect. Rationale for this includes its straightforward and minimally invasive technique, low cost, and low morbidity compared with other interventions. Usually performed arthroscopically, MF involves debridement of the defect to stable and vertical margins, removal of the calcified cartilage layer, and systematic penetration of the subchondral plate with an awl, drill, or pick ( Fig. 3 ). This technique achieves medullary bleeding, which releases undifferentiated mesenchymal stem cells and associated growth factors into the defect. As a stable, maximally filling clot has been correlated with success of this procedure, it is important that medullary bleeding be confirmed intraoperatively on tourniquet release. The technique requires that a sufficient number and depth of channels be produced without altering the subchondral osseous architecture. The clot matures to produce a repaired layer containing some type II cartilage but largely one of fibrocartilage characteristics, especially on analysis 1 year postoperatively.
The resultant fibrocartilage is a limitation of the technique, as long-term studies have reported inferior wear properties compared with hyaline cartilage and degeneration of this repaired layer with time. Although the fibrocartilage resulting from MF is inferior to native cartilage, a recent meta-analysis failed to show clear inferiority of MF to more advanced interventions, such as whole-tissue or cell-based techniques, as discussed later. Although there is a large body of data available within the knee literature, there still remains a paucity of long-term outcome studies following MF in other joints, such as the ankle and hip.
Most investigators agree with MF as the first-line surgical treatment of full-thickness defects less than 2.5 cm 2 in the knee, 1.5 cm 2 in the talus, and 4.0 cm 2 in the hip. A systematic review of 28 studies with more than 3100 knee patients reported function consistently much improved during the first 24 months postoperatively. More recently, 2 studies reported retained improvement and 7 reported deterioration in 47% to 80% of patients; however, postoperative functional scores still remained higher than preoperative scores. The 9 studies that included MRI reported highly variable degrees of cartilage filling the defect, with functional outcomes scores highly correlated with percentage of fill. Other identified factors predicting good outcomes included age less than 40 years, symptoms less than 12 months, defect less than 4 cm 2 , body mass index (BMI) less than 30 kg/m 2 , and no previous surgical intervention. Histology reports were varied, with a statistical trend toward fibrocartilage as compared with autologous chondrocyte implantation (ACI) ( P = .08). There was no statistical correlation between histology and clinical outcomes scores, but an association was found between improved histologic appearance and lower failure rate. Revision rates were significantly higher in more methodically strict studies, reported at a mean 8 to 38 months after MF.
Several studies have documented significantly better clinical outcomes following MF in younger patients, especially those younger than 40 years. Steadman and colleagues identified this in one of the first reports on long-term MF outcomes. In 71 knees followed for 11 years, all in patients aged less than 45 years, they found age to be an independent predictor of functional improvement.
MF in athletes has yielded similar results. Mithoefer and colleagues described deterioration of 47% of elite athletes’ knee function at an average of 41 months with only 44% returning to high-impact sports and 57% of these at their preoperative level. Schuman and colleagues reported that 45% of 38 patients with talar osteochondral defects were limited or unable to return to sport at a mean 4.8 years following marrow stimulation. Fourteen of 17 professional hockey players returned to play by 2 years following hip MF, though with decreased number of games played and points scored. With an increasing focus on utilization of health care dollars, Miller and colleagues recently analyzed 3 level 1 or 2 studies with 10-year follow-up, reporting lower initial cost with femoral condyle MF, followed by diminishing savings and overall near-equivalent cost with other techniques for return to play. Contributing to this were cumulative reoperation rates of 28.6% and 12.5% after MF and osteochondral autograft, respectively.
A recent systematic review of 12 arthroscopic hip MF reports including 267 patients with femoroacetabular impingement and 2.5-year follow-up indicated significant improvement for Outerbridge 4 defects. Variability in outcome measures prevented more specific analyses. Similarly, pooled analyses of talar MF is lacking, given individual study variability, though reported long-term outcomes at a mean of 10 years postoperatively have shown 65% of 82 patients with no or mild symptoms. Thirty-three percent have since increased one stage of radiographic arthritis.
Despite limitations of questionable durability and reports of a higher failure rate for cell-based techniques following a failed MF compared with primary cell-based intervention, MF remains the most commonly used first-line surgery. This point is especially true considering a lack of clear superiority of any one treatment thus far.
Based on a report of a significantly higher ACI failure rate when done after MF, caution is warranted in using marrow stimulation (with or without augmentation) for larger defects likely to require future ACI surgery, commonly with an area greater than 2.5 cm 2 .
Osteochondral autograft/mosaicplasty
Bone-to-bone healing has been shown to more reliably integrate than cartilage alone, making osteochondral transplantation an attractive option for chondral or osteochondral defects. Osteochondral autograft transplantation (OAT) is commonly referenced using various terminology, including the proprietary Osteochondral Autograft Transfer System (OATS) (Arthrex, Naples, FL) and mosaicplasty, the latter denoting transplantation of multiple grafts side by side in a mosaic-type fashion. OAT is a single-stage procedure of harvesting cylindrical osteoarticular plugs of healthy articular cartilage and underlying subchondral bone from an expendable area (typically lesser-weight-bearing portions of an ipsilateral femoral condyle) and transplanting to the symptomatic defect ( Fig. 4 ). It is performed both open and arthroscopically. As with microfracture, any unstable area is first debrided back to healthy margins and then measured to determine the size and number of plugs necessary. The transplanted grafts quickly incorporate to render a restored surface of viable, mature, hyaline cartilage. As one would expect, the single-stage nature and immediate restoration have produced more rapid improvement compared with ACI. Donor sites naturally heal with cancellous bone and overlying fibrocartilage.
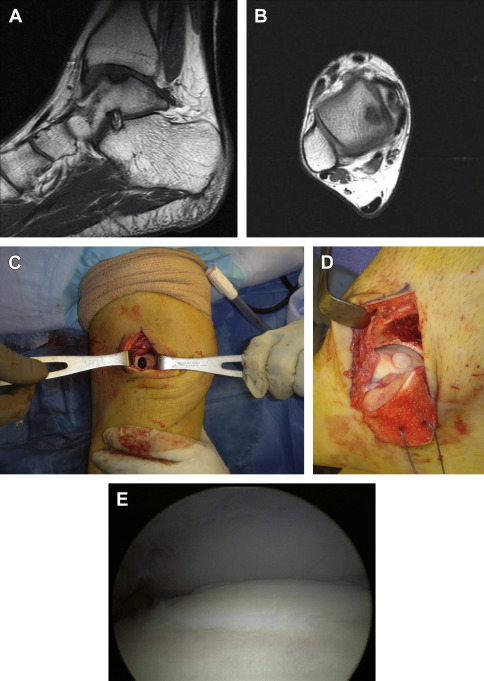
OAT indications include failure of bone marrow stimulation or primary use for relatively small to moderately sized areas (<3 cm 2 in knee) of focal cartilage loss with compromised underlying subchondral bone, including unstable osteochondritis dissecans lesions. Areas greater than 10 mm typically warrant multiple plugs.
Limitations include limited graft availability; donor-site morbidity; technical factors, including surgical exposure; and differences between donor and recipient joint cartilage, including thickness, contour, and mechanical properties. Reports of harvest-site knee pain have ranged from 0% to 37%. Plugs with depth matched to the defect will be more stable before integration and more likely to heal than a relatively short plug that relies on press-fit frictional forces alone. However, grafts left proud by 0.5 to 1.0 mm have shown a 50% increase in mean contact pressures, whereas those flush or slightly depressed restore near-normal contact pressures. Care must be taken to achieve the most congruent orientation possible. Integrity of the repair may also be hindered by potential dead space between plugs in mosaicplasty or from graft subsidence on weight bearing.
The exposure required for graft implantation in some locations is a limitation compared with the minimally invasive technique of MF and some cell-based techniques. Exposure for implantation can range from a mini-arthrotomy in the knee to plafondplasty or malleolar osteotomy in the ankle to surgical dislocation of the hip. Likewise, risks of these approaches range from relatively minor to potential for significant morbidity. These risks include arthrofibrosis, osteotomy nonunion, and osteonecrosis of the femoral head. These same approaches are commonly used for some cell-based techniques and allografting.
Long-term analysis of more than 1000 knee and talar mosaicplasty patients with small and medium-sized defects showed good to excellent clinical scores in 92% of femoral condyles, 87% of tibial lesions, 74% of patellar and/or trochlear defects, and 93% of talar implants. At least moderate donor-site complaints were reported in 3% of patients. Eighty-one of 98 follow-up arthroscopies showed congruent gliding surfaces with hyaline cartilage on histology at the transplant site and fibrocartilage at donor sites. Follow-up MRI reports vary, some with excellent fill and congruency within 1 mm in 84% of knees at mean 32.4 months. Other reports of quantitative T2 MRI talar analysis at a mean of 24.8 months have shown persistent differences from native controls.
Gudas and colleagues reported long-term results of a level 1 prospective randomized controlled trial comparing all-arthroscopic OAT to MF in knees of 60 elite athletes (mean age 24.3 years) with 95% follow-up at mean 10.4 years. The average lesion size was 2.8 cm 2 and included both osteochondral and articular-only lesions. Although both groups had decreased clinical scores compared with controls, both still showed significant improvement compared with presurgery scores. The OAT group had significantly better results at the final follow-up ( P <.005). Fourteen percent of OAT patients had required another operation compared with 38% of MF patients ( P <.05). The same physical activity level was maintained in 75% of OAT patients but only 37% of MF patients. MRI at 10 years showed complete defect filling in 75% of the OAT group and 35% of the MF group ( P <.05). Patients aged less than 25 years at the time of surgery showed persistently higher clinical scores at the final follow-up compared with older patients ( P <.05). Regardless of procedure, defects smaller than 2 cm 2 were associated with a significantly higher rate of return to sports ( P = .04). Although both techniques allowed a high rate of return to sports, these long-term results support a higher rate of return to preinjury athletic level, maintenance of activity, and lower failure rate with OAT in athletes.
A recent systematic review of OAT outcomes, including 607 patients in 9 level 1 and 2 studies, showed better clinical results from OAT/mosaicplasty when compared with MF, with better maintenance of preoperative activity level, higher rate of return to sports, and fewer reoperations. In comparing OAT with ACI, neither was clearly superior, though a greater failure rate at 10 years was found in the OAT group. The data suggested that OAT may show the most reliable benefit for lesions smaller than 2 cm 2 .
Osteochondral allograft
Osteochondral allografting (OCA) uses the same concept of osseous healing and immediate single-stage mature hyaline surface restoration as osteochondral autografting, with the added benefits of a cadaveric source and, therefore, no harvest-site morbidity, much-increased available donor area, and ability to precisely match architecture. OCA may be performed in a mosaicplasty fashion ( Fig. 5 ) as with OAT or as a bulk allograft ( Fig. 6 ). If performed in bulk, the resultant surface is uniform, avoiding potential dead space as with mosaicplasty, which allows use for defects of greater area as well as restoration of large areas of deficient underlying bone stock.
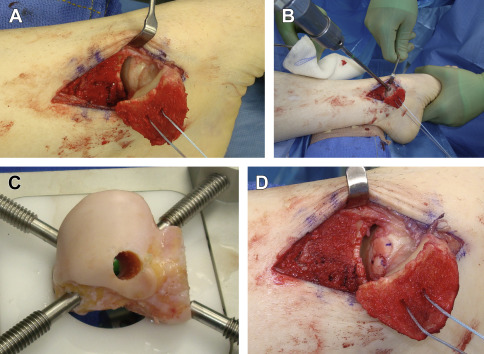
This open surgical technique can be used for a failed previous reparative procedure or as a bulk primary reconstruction for large defects (ie, >2.0–2.5 cm 2 ), including osteonecrosis with segmental collapse, large cystic areas or unreliable underlying structure, and localized posttraumatic arthritis. A substantial area may be replaced, including an entire femoral condyle in the knee, subtotal talar dome, and nearly half of the humeral head. When used in carefully selected patients, this technique has the potential to improve functionality and delay or possibly avoid arthrodesis or arthroplasty. OCA remains the only restorative option to address failed cell-based therapy or large osteochondritis dissecans lesions and also plays a vital role in reconstruction following tumor resection.
Specific techniques of OCA are joint dependent, but the general methodology involves first ensuring availability of a size-matched donor allograft. The lesion is debrided to clearly define its margins and is then resected. Great attention is given to matching the allograft implant to the resected specimen, in both size and orientation. The allograft is inserted with either a press-fit technique or internal fixation (see Fig. 6 ). The latter is more commonly used for larger grafts, including partial humeral head, talar dome, or knee hemicondyle.
Various storage methods have been investigated, with fresh allograft the most commonly used. Fresh-frozen and cryopreserved grafts have both demonstrated decreased chondrocyte viability on histologic analysis. Chondrocyte viability and incorporation of host bone directly correlate with the success of OCA. Other characteristics associated with favorable long-term outcomes include preservation of the extracellular matrix, robust mechanical stability at the time of implantation, and complete replacement of the graft with host bone, which occurs by creeping substitution. Fresh allografts are maintained in a temperature-controlled environment while undergoing a screening process, which must be both thorough and efficient, given that chondrocyte viability in fresh grafts has been shown to diminish with time. The surgeon and patients are both bound by this time-dependent relationship, requiring flexibility in surgical scheduling.
Limitations of OCA include the cost and availability of allograft tissue, chondrocyte viability following storage, size and contour matching, increased technical difficulty, and the potential for host rejection of the cadaveric graft.
Similar to the previously discussed techniques, available evidence suggests that patients most suitable for OCA are younger (<50 years), without evidence of adjacent osteoarthritis, with larger defects (ie, >2.0–2.5 cm 2 up to 10 cm 2 in large joints), or with substantial subchondral bone compromise. A defect of this nature can significantly influence quality of life in a younger population, in whom arthroplasty is a poor alternative. Likewise, MF and OAT have had suboptimal results in lesions greater than 2 cm 2 . ACI remains an option for larger lesions in the younger population, although it is multistage and less predictable with certain defect characteristics, such as an unshouldered talar lesion.
Levy and colleagues reported OCA efficacy for both primary and salvage intervention. One hundred twenty-nine knees (121 patients) undergoing femoral condyle OCA showed 82% survivorship at 10 years, 74% at 15 years, and 66% at 20 years. Similar results ranging from 84.5% to 100% at 5 years and 71% to 90% at 10 years have also been reported. In the series by Levy and colleagues, lesions stemming from corticosteroid-induced osteonecrosis produced worse results, a finding supported in other joints as well. Poor results also have been reported with bipolar, large (>10 cm 2 ), and increasingly chronic lesions and those in the context of primary osteoarthritis or inflammatory arthropathy.
Younger patients improve more predictably. Murphy and colleagues reported 39 OCA patients (43 femoral condyles) aged 18 years or younger. At 10 years, there was 90% survival with 88% good to excellent clinical outcomes scores and 4 of the 5 failures were salvaged with a revision allograft.
Fresh OCA is used frequently for large talar lesions (>1.5 cm 2 ) not suitable for other interventions, with moderate success in some subtotal dome defects. Raikin prospectively evaluated 15 patients after fresh allografting of cystic lesions with a volume of 3 cm 3 or greater at a mean follow-up of 44 months. Although clinical function and pain scores were improved, there were radiographic findings of graft resorption in 10 (67%) and joint space narrowing in 9 ankles (60%). Two (13%) required conversion to ankle arthrodesis.
More recently, Haene and colleagues reported allografting 17 ankles with a mean lesion volume of 3.4 cm 3 (range 1.0–10.9 cm 3 ). The mean follow-up was 4.1 years, with 10 (59%) reporting good to excellent outcome scores, 2 (12%) showing failure of graft incorporation on computed tomography, and overall 5 (29%) ankles considered failures, with 2 requiring reoperation.
Although these studies illustrate the guarded outcomes with use in large-volume talar lesions, this procedure provides a viable option to delay more permanent procedures, such as total ankle arthroplasty or arthrodesis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








