The spastic shoulder can often result from brain injury that causes disruption in the upper motor neuron inhibitory pathways. Patients develop dyssynergic muscle activation, muscle weakness, and contractures and often present with fixed adduction and internal rotation deformity to the limb. This article reviews the importance of a comprehensive preoperative evaluation and discusses appropriate treatment strategies based on preoperative evaluation.
Key points
- •
Spastic shoulder deformity can cause substantial limitation in active and passive upper extremity function.
- •
When deformities and lack of function persist and neurologic recovery has plateaued, preoperative planning should be initiated.
- •
The key is to characterize the patient’s motor control using a comprehensive physical examination, and when indicated, dynamic polyelectromyography and selective local anesthetic nerve blocks to identify the offending muscles.
- •
Treatment options include selective tendon releases, fractional tendon lengthenings, biceps suspension, arthrodesis, and arthroplasty, and must be determined after a careful review of the patient’s specific clinical presentation.
Introduction
Disorders of acquired spasticity most commonly include traumatic brain injury and cerebrovascular accidents and cause disruption of upper motor neuron inhibitory pathways. The result is upper motor neuron syndrome, which can manifest in spasticity, dyssynergic muscle activation, muscle weakness, and contractures. Most commonly, the patient’s shoulder assumes an adduction and internal rotation deformity, and this is the focus of this review. Patients can present with other patterns of deformity (eg, abduction, extension) depending on the pattern of muscle dyssynergy; however, these are beyond the scope of this review.
Patients with spastic shoulder deformity often describe substantial limitation in active and passive upper extremity function. When deformities and lack of function persist and neurologic recovery has plateaued, preoperative planning should be initiated to address residual spasticity and muscle contraction dyssynergy. The evaluation and surgical treatment of the spastic shoulder is uncommonly discussed. The determination of motor control necessitates a comprehensive physical examination and often includes dynamic polyelectromyography (poly-EMG) and selective local anesthetic nerve blocks to identify the offending muscles. Corresponding shoulder disease, including glenohumeral subluxation and arthrosis, must also be identified and treated. Treatment options include selective tendon releases, fractional tendon lengthenings, biceps suspension, arthrodesis, and arthroplasty, and must be determined after a careful review of the patient’s specific clinical presentation.
Upper Motor Neuron Syndrome
Upper motor neuron syndrome is defined as the change in motor control that occurs after an upper motor neuron injury, such as a cerebrovascular accident, spinal cord injury, traumatic brain injury, or other acquired central nervous system injury. Characteristics of upper motor neuron syndrome include the presence of spasticity and other forms of involuntary muscle overactivity, voluntary weakness, and a variety of motor control abnormalities that impair the regulation of voluntary movement. Patients with upper motor neuron lesions can present with highly variable combinations of impaired voluntary movement and involuntary muscle contractions. Of these 2 defects, it is the spastic and involuntary muscle contractions that may be painful and debilitating. Seventeen percent to 48% of patients who suffer a cerebrovascular accident are affected by spasticity, and approximately one-third of patients with traumatic brain injury experience some level of limb spasticity. With the annual number of stroke at 780,000 and a combined 1.7 million new spinal cord and traumatic brain injuries in the United States each year, the sequelae of upper motor syndrome are a source of substantial morbidity, which require skilled medical treatment.
In the early stages of upper motor neuron syndrome, the imbalance of neurologic signals results in sustained muscle contraction found in the stretch reflex. In normal neuromuscular physiology, the stretching of a muscle body causes the muscle spindle Ia afferent neurons to excite motor neurons, which respond with contraction of agonist and relaxation of antagonist muscles. The result of this balance is muscular tone, which allows the body to control itself within space and resist or influence forces within its environment. When an upper motor neuron injury occurs, the balance is shifted toward excitatory inputs, which result in motor neurons with lower threshold potentials and longer discharges. As a result, patients often experience unopposed muscle contraction or dyssynergic muscle activity. In this clinical scenario, the normal stretch reflex that exists to protect the body from outside forces acts as a debilitating factor. Continued contractions lead to changes within the muscle tissue itself, as fibrous and adipose tissue begin to replace the normal contractile sarcomeres. Remaining sarcomeres become shortened as a result of their constantly contracted state, and normal elastic tissue is lost. These sustained muscle contractions can lead to stereotypical postures of the various joints, namely the adduction and internal rotation deformity seen most commonly in the shoulder.
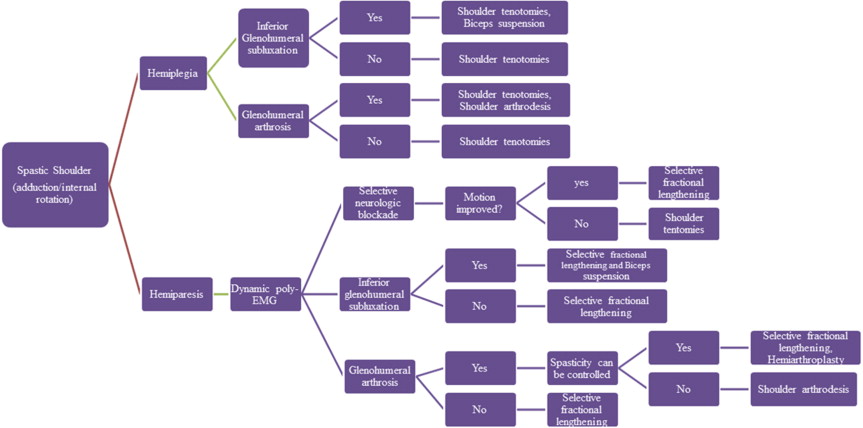
Patients with spastic shoulders after upper motor neuron injury can either lack all motor control (hemiplegia) or have variable levels of preserved motor control (hemiparesis). This is a critical distinction in the evaluation of the spastic shoulder and one that is underscored throughout this review. Based on the degree of preserved motor control, the clinical picture often involves a combination of active and passive functional problems. Regardless of the specific patterns of spasticity and muscle overactivity, treatment should begin with nonoperative intervention aimed at preventing and treating the passive and dynamic components of spasticity.
Nonoperative Treatments
Medications
Nonoperative treatments for shoulder spasticity include oral medications, which have the benefit of being noninvasive. The most common targets of antispasmodic medications are the γ aminobutyric acid (GABA) system, the α 2 adrenergic system, and the sarcoplasmic reticulum calcium release system within muscle tissue. Baclofen and benzodiazepines act as agonists on GABA-B and GABA-A receptors, respectively, and are helpful in decreasing the release of excitatory neurotransmitters responsible for firing motor neuron signals. These medications are not without side effects, because both can cause drowsiness, and baclofen can cause urinary incontinence, sexual dysfunction, and lower seizure threshold. Even more concerning is the potential for rebound spasticity seen within 48 hours of discontinued use of baclofen. Despite these concerns, baclofen remains the preferred drug treatment of spasticity and can be administered orally or intrathecally.
Tizanidine is an ∞ 2 receptor agonist that inhibits excitatory pathways in the spinal cord and brain by enhancing noradrenergic activity. One of the benefits of this drug is that it has enhanced tolerability compared with the GABA agonists. Side effects include dry mouth and gastrointestinal irritation. Tizanidine should be avoided in patients with hypotension because it can exacerbate this condition. Its most concerning side effect is acute hepatitis, which must be accounted for with early liver enzyme monitoring.
Dantrolene is commonly known as a treatment in the acute setting of malignant hyperthermia caused by general anesthesia. It works by blocking the calcium release from the sarcoplasmic reticulum, which causes muscle cell contraction and interferes with the excitation-contraction coupling seen in muscle tissue. Unlike the medications mentioned earlier, dantrolene works directly on muscle tissue and is less likely to cause sedation and dizziness. However, it does also require liver enzyme monitoring because of risk of acute hepatitis.
Physical therapy
Physical therapy can offer a gradual and long-term approach to maintaining range of motion. It is typically an integral adjunct of the nonoperative and surgical treatment of spastic patients throughout their lifetime. The key to the physical therapy treatment of a patient with upper motor neuron syndrome is to start as soon as possible and to continue as long as possible. Passive stretching helps to decrease the excitatory threshold of the motor neurons and prevent the loss of elastic connective tissue. This treatment can be labor intensive for the patient and the therapist. Exercise machines can often take the place of a physical therapist. Posteraro and colleagues reported on a regimen of active robot-assisted training, which avoids shoulder flexor patterns (active shoulder adduction and internal rotation movements) and which showed improved shoulder and elbow motor function in poststroke participants after 3 months of intervention. Overly aggressive passive range of motion can cause injury to soft tissue or bone, resulting in heterotopic bone formation. Spastic patients often have concomitant disuse osteopenia of the involved extremity, and so, an internal rotation contracture makes a person vulnerable to a spiral fracture of the humerus with even gentle manipulation. As a result, it is important for the physical therapist to be aware of a patient’s limitations and contour therapy regimens to their abilities without causing iatrogenic harm. Furthermore, it is important to recognize when gains in motion with physical therapy have plateaued and surgery is advisable rather than continued, or more aggressive, manipulation.
Chemodennervation
The 2 most common injection treatments for spasticity are botulinum toxin and phenol. Botulinum toxin is prepared from the Clostridium botulinum bacterium and works by binding to presynaptic nerve endings and blocking the release of acetylcholine. This treatment prevents the motor neuron signals from carrying out their excitatory inputs on muscle tissue. This paralytic toxin can target specific muscle groups through carefully placed injections. In a randomized, double-blind trial of 37 poststroke patients with shoulder spasticity, the injection of botulinum toxin was found to improve hygiene and Disability Assessment Scale scores compared with placebo injections of saline. However, the same injections offered no improvement in pain scores for patients. Such results emphasize the importance of a multiple modality approach to nonoperative therapy. In addition, the effects of botulinum toxin injections disappear as new nerve endings grow and reinnervation of specific muscle groups occurs over a few months. Patients who undergo this treatment should be reassessed every 4 to 6 weeks and offered repeat injections if success is met with previous injections.
Phenol is another injection available for the treatment of spasticity. It causes local destruction of peripheral neural tissue. Phenol blocks are especially effective for powerful muscle groups that cause limb deformity and are performed by physicians skilled in ultrasound guidance. A study of 13 hemiplegic patients with shoulder spasticity showed that phenol injection of the subscapular nerves afforded patients immediate improvement in range of motion and pain relief. Similarly, phenol injections of the pectoralis major have been used with success in reducing shoulder spasticity. However, injection of a mixed motor sensory nerve with caustic agents, such as phenol, can cause complex regional pain syndrome or muscle fibrosis and are no longer commonly performed. Intramuscular injections of botulinum toxin do not cause this problem but have the disadvantage of being temporary.
Preoperative Evaluation of the Spastic Shoulder
Physical examination
The preoperative evaluation should begin with an understanding of the patient’s level of cognitive impairment and social support system. Both of these variables are important in dictating a patient’s ability to comply with postoperative instructions and rehabilitation. Cognitive impairment, learning ability, and short-term memory can often be evaluated by the appropriateness of a patient’s response to questions, ability to follow commands, psychological testing, and by direct testing of learning ability in a rehabilitation setting. Patients being considered for surgery have often failed physical therapy, and feedback from the physical therapist and caretakers can be helpful in understanding the patient’s cognitive abilities. Aphasia is the loss of ability to communicate properly and can be expressive or receptive. Receptive aphasia is associated with a poor prognosis for rehabilitation, because the patient cannot understand instructions. However, a patient with expressive aphasia may be able to undergo rehabilitation because they are able to understand and follow instructions. Presence of apraxia, an inability to perform a learned movement in the absence of motor impairment, is an important consideration when discussing expectations of surgery. Patients with apraxia should be counseled that the prognosis for improvement in active motor function is poor. It is necessary to understand a patient’s social support system, including family and caregivers, because these members can be important in clarifying a patient’s active or passive goals of treatment; the social support system is important in the postoperative recovery process.
As noted earlier, patients with spastic shoulders after upper motor neuron injury can either lack all motor control (hemiplegia) or have variable levels of preserved motor control (hemiparesis). In evaluating the spastic shoulder, it becomes crucial to identify the clinical pattern of motor function, characterize the patient’s ability to control their muscles, and the influence of stiffness and contractures on both exacerbating symptoms and masking intact motor control. A fixed limb deformity may be caused by severe spasticity or alternatively by soft tissue contracture of muscles, tendons, and ligaments. Differentiating between these 2 causes can be clinically challenging. Like all joints, the glenohumeral joint is traversed by agonist and antagonist muscles that can facilitate movement and stabilize the joint in patients with normally functioning anatomy or can result in patterns of dyssynergy and dysfunction in those with upper motor neuron injury. Through clinical evaluation, the physician can observe asymmetry in form or movement, palpate muscles for tone and spasticity, and assess muscle strength. Along with testing passive range of motion, the ability to actively initiate movement and maintain the arm in space is important to identify clinically. Contractures are common, and the shoulder is often held in an adducted and internally rotated posture. Triceps, biceps, and brachioradialis reflexes should be tested with a reflex hammer to confirm presence of spasticity. Spasticity can be measured by resistance to passive movement and graded by the Ashworth scale. With this scale, 0 means no increase in tone (none); 1 means a slight increase in tone, giving a catch when the limb is moved in flexion or extension (mild); 2 means a more marked increase in tone but the limb is easily flexed (moderate); 3 means a considerable increase in muscle tone (passive movement is difficult) (severe); and 4 means the limb is rigid in flexion or extension (very severe). In cases of inferior glenohumeral subluxation, a sulcus sign is often encountered, and leaving the arm unsupported may be painful for the patient. If a patient’s symptoms are relieved by manual reduction of the subluxation, the pain is considered mechanical in nature and potentially amenable to surgical stabilization.
A thorough evaluation of the neurologic status of the limb is important, because patients present with various levels of impaired sensibility. Sensibility to light touch, as well as 2-point discrimination, is an important preoperative consideration. Patients with significant sensory impairments and a lack of protective sensation may be poor candidates for surgical intervention. Proprioception, a patient’s understanding of the position of a limb in space, is also important to determine preoperatively. Proprioception can be determined by the up-or-down test at the distal interphalangeal joint. Lack of proprioception does not represent a contraindication to surgery; however, like sensibility, it is not improved by neuro-orthopedic intervention and should be discussed with patients to generate realistic expectations regarding surgical outcomes.
Imaging/advanced testing
Radiographs
Evaluation of the spastic shoulder should always include plain radiographs (anterior-posterior view, scapular-Y view, and axillary view). Spasticity and contractures that create a rigid adduction deformity may prevent acquisition of an axillary view, and a Velpeau view may be more appropriate. Radiographs are important to rule out subluxation of the humeral head, heterotopic ossification, or joint arthrosis, which can contribute to stiffness. Advanced imaging techniques, such as magnetic resonance imaging or computed tomography, are often unnecessary unless one of the diseases mentioned earlier is present.
Dynamic poly-EMG
Historically, clinical examination has been the mainstay of evaluation and decision making for patients who have spastic limb deformities. Clinical assessment, supplemented by instrumented laboratory analysis with dynamic poly-EMG, has helped characterize movement disorders and has been shown to improve the outcomes of treatment. In our practice, dynamic poly-EMG is used in cases in which there is preserved motor control and the surgical preference is for selective tendon lengthening rather than tendon/muscle releases. As noted earlier, in patients who show volitional control in the limb, the major clinical question is whether the limited shoulder motion is a result of absent or weak muscle activity or the result of inappropriate activity (dyssynergy or cocontraction) of the antagonist muscles. For example, an inability to actively externally rotate the shoulder may be a result of weakness in the shoulder external rotators or a problem with dyssynergic activity of the internal rotators during attempted external rotation. In this situation, dynamic poly-EMG may reveal a normally active posterior deltoid during attempted external rotation but dyssynergic activity of the pectoralis major and latissimus dorsi ( Fig. 1 ). As a result, a hypothesis can be generated that lengthening, or effectively weakening, the pectoralis major and latissimus dorsi could improve active external rotation.

During dynamic poly-EMG testing, sensors are placed on multiple muscles, and information is recorded while the person is moving. EMG recordings and movement tracings are often obtained from specific muscles, including the lateral head of the triceps, pectoralis major, teres major, and latissimus dorsi during passive motion by the examiner and attempted active motion by the patient. Poly-EMG data can interpret whether effort-related initiation, modulation, and termination of voluntary activity are present in a given muscle. Poly-EMG can also identify dyssynergy or cocontraction, defined as inappropriate muscle firing during antagonist motion. If a patient has preserved motor control in agonist muscle groups, weakening antagonist muscles that are dyssynergic by fractional lengthening can result in improved active function.
Selective neurologic blockade
Selective neurologic blocks can be used as an adjunct to the diagnostic evaluation and preoperative planning of patients with spastic shoulder disease. When cocontraction is present in a muscle with appropriate agonist activity, it is thought that removing the dyssynergic activity results in improved volitional motion. However, if contractures are too rigid, fractional lengthening may be ineffective in sufficiently tempering the effects of the antagonist muscle and a tendon release may be necessary. Although EMG may show motor function in the posterior deltoid and dyssynergic activity in the internal rotators during attempted external rotation, fractional lengthening may not yield a substantial gain in external rotation if severe contractures of the musculotendinous units of the pectoralis major and latissimus dorsi are present. In cases in which there is a question regarding whether contractures are too rigid to result in substantial recovery of active motor function with fractional lengthening, selective bupivacaine blocks of dyssynergic muscles can be used to temporarily relieve spasticity and to show improvement in motion. Deformity caused by spasticity improves after a nerve block, but deformity caused by a fixed contracture does not change after administration of local anesthetic.
Surgical Treatment of the Spastic Shoulder
Shoulder tenotomies
Shoulder tenotomies are appropriate to consider in patients with upper motor neuron disease without voluntary control of their limb (hemiplegia) ( Fig. 2 ). These contractures can cause pain and interfere with axillary hygiene and activities of daily living. Although the limb may be functionless, improving the position of the arm may improve the function of the patient by making self-care and caregiver-assisted care, such as dressing and axillary hygiene, easier. For example, making a shoulder passively flexible can result in a hemiplegic patient becoming independent in upper body dressing. Previous EMG studies have shown that the adduction and internal rotation deformity is caused by spasticity and myostatic contracture of the pectoralis major, latissimus dorsi, teres major, and subscapularis muscles. Unless there is a question regarding the presence or absence of active motor control on preoperative physical examination, we do not routinely obtain dynamic poly-EMGs of patients without preserved motor control. In the nonfunctional extremity, all 4 involved muscles should be released.
This technique has been previously described. The patient is positioned supine on a hand table with a bolster under the scapula, or in a modified (30°–45°) beach chair position. A full beach chair is avoided because of commonly encountered medical comorbidities in these patients and concern for adequate cerebral perfusion. A 7-cm incision is made in the anterior portion of the shoulder approximating the deltopectoral interval. The cephalic vein is identified and retracted laterally with the deltoid. In the distal portion of the interval, the sternal and clavicular heads of the pectoralis major are identified along the lateral border of the intertubercular groove of the humerus and released. The subscapularis tendon is then identified and carefully tenotomized from the lesser tuberosity, taking care to avoid violating the underlying glenohumeral joint capsule. Dissection is then carried deep and medial. The brachial plexus and axillary artery are retracted carefully. The tendon insertion of the latissimus dorsi is identified just distal to the muscular portion of the subscapularis and medial to the intertubercular groove and is also released from the humerus. The arm can be further externally rotated, and the teres major tendon can be identified deep to the latissimus dorsi insertion and also released. A drain is placed in the deep wound before closure to minimize postoperative hematoma.
Postoperative rehabilitation is critical to maintain correction and decrease the possibility of recurrence. The limb should be placed in slight abduction and external rotation. This position can often be simply achieved by patients with several pillows placed at the side. Self-assisted passive range-of-motion exercises are started immediately on the first postoperative day, including supine forward elevation, external rotation, and horizontal abduction. In some cases, the skin may be too frail to begin immediate range of motion. In these cases, the patient is immobilized in a favorable position (slight abduction and external rotation) and range of motion is started after 2 to 3 weeks.
We studied 36 hemiplegic patients who underwent releases of the pectoralis major, subscapularis, latissimus dorsi, and teres major for adduction and internal rotation contractures after upper motor neuron injury. Preoperative indications for surgery were pain, and difficulty with dressing, skin care, or hygiene. Average follow-up was 14.3 months. Although 53% of patients reported preoperative pain, all noted improved pain relief, with 95% being pain free at final follow-up. Patients showed significant improvements in passive range of motion in extension (20°–34°), flexion (49°–125°), abduction (43°–105°), and external rotation (1°–45°). All patients noted improvement in hygiene, skin care, and caregiver-assisted dressing, and 97% were satisfied with the outcome of surgery. There were 2 hematomas that resolved without intervention, and no postoperative infections or neurovascular injuries.
Shoulder fractional lengthenings
Hemiparetic patients with selective motor control (hemiparesis) have the potential to achieve a greater degree of function, because some level of active motion is preserved. These patients often show a flexion synergy pattern, in which the spastic flexors and internal rotators are mass activated when the patient attempts to perform a functional task. In this population, functional limitations can be a result of either contracted muscles or abnormal patterns of activation and spasticity. The clinical question is often whether the lack of function observed is a result of weakness of agonist muscles or inappropriate activation of antagonist muscles. As noted earlier, dyssynergy or cocontraction in antagonist muscle groups may be inhibiting functional agonist muscle activity. In theory, if antagonist muscle groups are creating a mass activation pattern that results in loss of function, selectively lengthening these muscles should produce functional improvement. In this subset of patients, we routinely obtain dynamic poly-EMG studies to clarify the clinical picture and to generate a plan for selective tendon fractional lengthening. The most commonly involved muscles are the pectoralis major, latissimus dorsi, teres major, and long head of the triceps, which act in cocontraction with activation of the shoulder flexors during attempted flexion.
This technique has been previously described. The patient may be positioned as described in the shoulder tenotomy technique. Again, a standard deltopectoral incision is made. The goal of fractional lengthening is to cut the tendon at the musculotendinous junction as it overlaps the muscle. When performed in this manner, the tendon lengthens based on the amount of spasticity in the limb. The undersurface of the pectoralis major must be exposed, because the tendinous portion is found on the undersurface of the muscle. Enough proximal exposure must be available to identify the musculotendinous portions of the latissimus dorsi, teres major, and long head of the triceps in the deep portion of the wound near the medial humerus. The triceps tendon may be found deep to and superior to the tendinous portions of the latissimus dorsi and teres major. The brachial plexus must be retracted medially. Meticulous hemostasis must be obtained, followed by placement of a drain.
No immobilization is generally required. When the patient is resting, a pillow is recommended at the side to allow the tendon to heal in a lengthened position and to prevent recurrence. Active and active-assistive range of motion exercises are initiated on the first postoperative day. Resistive exercises and passive stretching are avoided for 3 to 4 weeks to avoid rupture of the lengthened muscles.
We reported our experience with 34 hemiparetic patients who underwent selective fractional lengthening for spastic shoulder contractures after upper motor neuron injury. All patients had difficulty with activities of daily living, including skin care, hygiene, and dressing. All patients underwent lengthening of the pectoralis major, latissimus dorsi, and teres major. In addition, 4 patients underwent lengthening of the long head of the triceps based on findings on dynamic poly-EMG. At mean 1 year of follow-up, there were significant improvements in active flexion (59°–116°), abduction (51°–95°), and external rotation (6°–45°). Of patients who had pain preoperatively, 88% were pain free and 92% were satisfied with the outcome of surgery. One patient who did not comply with postoperative instructions experienced a recurrence and did not have pain relief.
Inferior glenohumeral subluxation
After an injury involving a hemisphere of the brain, there is flaccid paralysis contralateral to the involved side. During this period of flaccid paralysis, a subluxation of the glenohumeral joint often occurs. This subluxation is most commonly self-limiting and asymptomatic. During the flaccid paralysis stage and before the onset of spasticity, a sling to support the arm or functional electrical stimulation to maintain the glenohumeral joint in a reduced position can be an important means of prophylaxis against persistent subluxation as spasticity develops. In some patients, painful subluxation of the glenohumeral joint and corresponding spasticity persist. If the pain is relieved by manual reduction of the glenohumeral joint, and alleviated by support of the limb in a sling, surgical correction of the subluxation may be considered. The biceps suspension procedure is our technique of choice for this condition. Dynamic poly-EMG is performed if other sequelae of upper motor neuron disease with preserved motor control are being treated concomitantly. If the biceps suspension procedure is unsuccessful, a shoulder arthrodesis is also a possible surgical treatment option.
The biceps suspension technique has been previously described. The patient is positioned supine with the arm resting on a hand table. In general, supine positioning reduces the glenohumeral joint without the need for any proximally directed force. A standard deltopectoral approach is used. Lengthenings or releases of the adductor/internal rotator muscles are performed as dictated by the preoperative plan. The tendon of the long head of the biceps is identified in the intertubercular groove. The biceps tendon is dissected from the groove, but the rotator interval is not violated. The biceps is then cut at its musculotendinous junction, and all muscle fibers are sharply removed from the tendon. A Krackow stitch using a stout suture such as number 2 Fiberwire (Arthrex, Naples, FL) is placed at the distal end of the biceps tendon, tubularizing it. The intertubercular groove is then subperiosteally exposed, and 2 5-mm drill holes, 1 proximal and 1 distal in the groove, are made (approximately 2 cm apart). A curved curette is used to connect the 2 holes, with care not to break the bone bridge between the drill holes. A suture passer or free needle is used to pass the tendon through the drill holes from proximal to distal. Traction is placed on the tendon to reduce the subluxation. The biceps is then sewn to itself with a number 2 Fiberwire. Meticulous hemostasis is obtained, and a deep drain is placed. The repair is protected in a sling for 3 months. Gentle self-assisted passive range of motion can be started on the first postoperative day, but the arm should be supported at all times to prevent strain on the repair.
Our experiences with this procedure have been described in a series of 11 patients at minimum 2-year clinical follow-up. Nine patients had complete reduction of the subluxation on postoperative radiographs; 1 had a partial reduction; and 1 had recurrent subluxation. Nine patients were satisfied with the outcome of surgery. All patients noted a decrease in pain, and 10 patients reported an improvement in the appearance of their shoulder.
Shoulder arthrosis/arthroplasty
The combination of increased tone in the shoulder musculature, contractures, and glenohumeral arthrosis can be a challenging clinical problem. A major clinical question remains whether the patient has preserved motor control. If the patient is hemiplegic and lacks motor control, a shoulder arthrodesis is often the most reliable treatment option for pain relief and repositioning of the limb in space. During fusion, contracture releases can allow for positioning of the limb in an appropriate position for fusion. In general, we place the shoulder in 20° of flexion, 30° of abduction, and 40° of internal rotation. It is critical to provide sufficient abduction to allow for axillary hygiene. No study has specifically evaluated the outcomes of shoulder fusion in patients with upper motor neuron injury. Thin skin and muscle atrophy can lead to postoperative wound complications from prominent hardware, because plates often rest below the skin surface. Skin may require Z-plasty during closure, hardware should be minimized when possible, and local muscle flaps should be considered for particular complex cases.
If the patient is hemiparetic and has some level of preserved motor control, the secondary clinical question is whether spasticity can be controlled sufficiently to allow for stability of a shoulder arthroplasty. The pain of the surgery can often produce increased muscle tone in the acute postoperative setting, thus creating a cycle of pain and spasticity that can put the arthroplasty at risk for instability. As a result, we generally avoid placement of a glenoid implant because of theoretic concern of early loosening and wear from eccentric loading resulting from shoulder spasticity. Hattrup and colleagues described 3 cases of total shoulder arthroplasty in patients with upper motor neuron injury and glenohumeral arthrosis. Although arthroplasty was effective in relieving pain in all 3 patients, 2 patients experienced postoperative subluxation. In their small series, the 1 patient without postoperative subluxation of the glenohumeral joint underwent botulinum toxin injections in muscles selectively chosen after assessment of the patient’s dystonic pattern on physical examination. In our experience, patients with preserved active motor control and glenohumeral joint arthrosis can benefit from shoulder hemiarthroplasty, if combined with appropriate treatment of shoulder spasticity. The surgeon should not allow the presence of arthrosis to distract from the standard workup of spasticity. In cases of preserved motor control, a dynamic poly-EMG should be obtained; and if necessary, selective neurogenic blockade can be helpful in fully delineating patterns of spasticity. At the time of arthroplasty, appropriate muscle fractional lengthenings and botulinum injections (ie, deltoid) for muscle groups that cannot be lengthened are important to control postoperative spasticity and joint stability. When inferior glenohumeral joint subluxation is also present, a biceps suspension procedure can be similarly added to the treatment plan of hemiarthroplasty and selective fraction lengthenings.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





