Abstract
Optogenetics is the combination of optical tools to monitor (i.e. “reporters”) or interfere (i.e. “actuators”) with neural activity, and genetic techniques to restrain the expression of these reporters and actuators in the neuronal populations of interest. Such combination of optical and genetic tools, together with the emergence of new animal models such as the zebrafish larva, has proven extremely valuable is dissecting neural circuits. Optogenetics provide a new framework to address issues that are fundamentally dynamic processes, such as sensorimotor integration in the vertebrate spinal cord. By shifting from spatially targeted electrical stimulation to genetically targeted optical stimulation, optogenetic also opens new avenues for innovative neurorehabilitative strategies, in particular after spinal cord injury.
1
Introduction
A critical step in order to understand neural circuits is the ability to selectively monitor and interact with populations of neurons. Until recently, electrophysiological techniques allowed recording from and stimulating only a limited number of morphologically identified neurons from ex-vivo tissue samples or paralyzed animals. Optogenetics, which is the combination of optical tools and genetic targeting techniques, has revolutionized this paradigm by allowing both monitoring and stimulation or inhibition of genetically identified populations of neurons, in paralyzed but also in moving animals.
Optogenetic neuromodulation relies on optical “reporters”, which emit light when the cell is active, to inform us on the activity of the population of neurons that has been genetically targeted. On the other hand, optical “actuators”, which have the ability to depolarize or hyperpolarize the neurons in which they are expressed, allow us to manipulate neural circuits with light. The combination of optical reporters and actuators with advanced genetic techniques to target their expression in precise populations of neurons provides new avenues to break neural circuits and ultimately understand their function in intact vertebrates.
2
Reporters: monitoring neural circuits
Monitoring neural activity can be indirectly achieved by measuring the intracellular level of calcium, since electrical activity of neurons lead to a calcium influx through voltage dependent calcium channels . This strategy has led to the elaboration of number of chemical calcium indicators and genetically encoded calcium indicators (GECI) that have been successfully used in many different mammalian and non-mammalian animal models ( Fig. 1 A1). GECIs consist in engineered fluorescent proteins having two key features: their emission properties are modified depending upon the intracellular level of calcium, and their pattern of expression can be restricted using the above mentioned genetic toolbox. They include either permutated single fluorescent proteins whose fluorescence properties are modified when calcium is binding to Ca 2+ recognition elements , or pairs of fluorescent proteins in which conformational change induced by calcium binding leads to Förster Resonance Energy Transfer (FRET) mediated modification of fluorescence .
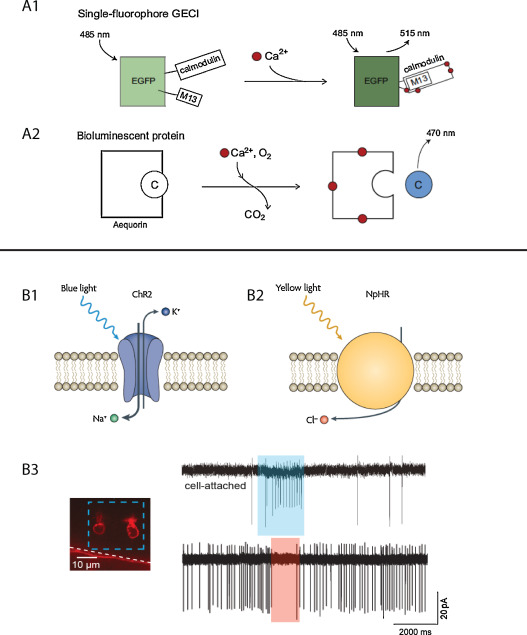
The transparency of the zebrafish larva and its genetic accessibility make it an ideal model to use such optical tools for monitoring neural activity. In the first zebrafish study using a GECI (cameleon), expressed under the islet-1 promoter (see section 3.1.2), calcium transients could be observed within the spinal cord, in Rohon-Beard neurons activated by electrical cutaneous stimulation, and in motoneurons and Commissural Descending (CiD) interneurons during escapes triggered by a mechanical head tap . Since this first study, GECIs have been extensively used in zebrafish to monitor neural activity in various behavioral paradigms, including investigating the role of the optic tectum in prey capture , performing brain-wide monitoring of neural dynamics in a sensorimotor virtual environment or testing neural coding of odors by the olfactory bulb . Targeted mutagenesis and high-throughput screening have led to the continuously improvement of GECIs such as the single-fluorophore GCaMP family by optimizing their calcium affinity, kinetics and dynamic range . From the first GCaMP to the current GCaMP6 , and including the generation of multi-color variants , the always improving GECIs arsenal now allow for monitoring of neural activity over a wide range of firing rates.
One major limitation of GECIs such as GCaMP, regarding in particular investigation of closed-loop sensorimotor behaviors in vivo, is the need for providing focal excitation to the fluorescent proteins. Indeed, this limitation implies constraining the neurons of interest to a given focal plane, either by partially embedding and/or paralyzing the animal. One alternative approach is to use the bioluminescent protein-aequorin-GFP, derived from the jellyfish Aequorea victoria ( Fig. 1 A2). ApoAequorin, the naturally occurring complex of aequorin with GFP, binds to its substrate coelenterazine, which is then oxidized in the presence of calcium leading to the emission of a green photon by the GFP through chemiluminescence resonance energy transfer (CRET) . Bioluminescence assays based on aequorin-GFP have been used for noninvasive monitoring of neural activity in vitro , but also in restrained flies and freely behaving mice .
Taking advantage of this bioluminescence approach, monitoring of neural activity in freely behaving zebrafish larvae has been achieved by genetically targeting the expression of aequorin-GFP in a specific subset of neurons and simultaneously counting the number of photons emitted over time while recording the locomotor activity using a high-frequency camera . Remarkably, the author could monitor the activity of a small group of hypocretin-positive neurons in the hypothalamus over several days, or combine a gated photomultiplier tube with stroboscopic illumination to record visually evoked behaviors . While the aequorin allows for noninvasive monitoring of an entire population of neurons in a moving animal, it does provide any spatial information, thus making the specificity of the genetic targeting a crucial limitation.
2
Reporters: monitoring neural circuits
Monitoring neural activity can be indirectly achieved by measuring the intracellular level of calcium, since electrical activity of neurons lead to a calcium influx through voltage dependent calcium channels . This strategy has led to the elaboration of number of chemical calcium indicators and genetically encoded calcium indicators (GECI) that have been successfully used in many different mammalian and non-mammalian animal models ( Fig. 1 A1). GECIs consist in engineered fluorescent proteins having two key features: their emission properties are modified depending upon the intracellular level of calcium, and their pattern of expression can be restricted using the above mentioned genetic toolbox. They include either permutated single fluorescent proteins whose fluorescence properties are modified when calcium is binding to Ca 2+ recognition elements , or pairs of fluorescent proteins in which conformational change induced by calcium binding leads to Förster Resonance Energy Transfer (FRET) mediated modification of fluorescence .
The transparency of the zebrafish larva and its genetic accessibility make it an ideal model to use such optical tools for monitoring neural activity. In the first zebrafish study using a GECI (cameleon), expressed under the islet-1 promoter (see section 3.1.2), calcium transients could be observed within the spinal cord, in Rohon-Beard neurons activated by electrical cutaneous stimulation, and in motoneurons and Commissural Descending (CiD) interneurons during escapes triggered by a mechanical head tap . Since this first study, GECIs have been extensively used in zebrafish to monitor neural activity in various behavioral paradigms, including investigating the role of the optic tectum in prey capture , performing brain-wide monitoring of neural dynamics in a sensorimotor virtual environment or testing neural coding of odors by the olfactory bulb . Targeted mutagenesis and high-throughput screening have led to the continuously improvement of GECIs such as the single-fluorophore GCaMP family by optimizing their calcium affinity, kinetics and dynamic range . From the first GCaMP to the current GCaMP6 , and including the generation of multi-color variants , the always improving GECIs arsenal now allow for monitoring of neural activity over a wide range of firing rates.
One major limitation of GECIs such as GCaMP, regarding in particular investigation of closed-loop sensorimotor behaviors in vivo, is the need for providing focal excitation to the fluorescent proteins. Indeed, this limitation implies constraining the neurons of interest to a given focal plane, either by partially embedding and/or paralyzing the animal. One alternative approach is to use the bioluminescent protein-aequorin-GFP, derived from the jellyfish Aequorea victoria ( Fig. 1 A2). ApoAequorin, the naturally occurring complex of aequorin with GFP, binds to its substrate coelenterazine, which is then oxidized in the presence of calcium leading to the emission of a green photon by the GFP through chemiluminescence resonance energy transfer (CRET) . Bioluminescence assays based on aequorin-GFP have been used for noninvasive monitoring of neural activity in vitro , but also in restrained flies and freely behaving mice .
Taking advantage of this bioluminescence approach, monitoring of neural activity in freely behaving zebrafish larvae has been achieved by genetically targeting the expression of aequorin-GFP in a specific subset of neurons and simultaneously counting the number of photons emitted over time while recording the locomotor activity using a high-frequency camera . Remarkably, the author could monitor the activity of a small group of hypocretin-positive neurons in the hypothalamus over several days, or combine a gated photomultiplier tube with stroboscopic illumination to record visually evoked behaviors . While the aequorin allows for noninvasive monitoring of an entire population of neurons in a moving animal, it does provide any spatial information, thus making the specificity of the genetic targeting a crucial limitation.
3
Actuators: breaking neural circuits
Besides monitoring neural activity, the optical and genetic accessibility of the zebrafish larva also constitute an optimal playground for optogenetic actuators, making it possible to selectively activate or inhibit genetically targeted neurons . Channelrhodopsin-2 (ChR2) is a light-gated channel derived from the unicellular alga Chlamydomonas reinhardtii allowing non-specific influx of cations when illuminated with blue light ( Fig. 1 B1). ChR2 can therefore be used to control a genetically targeted neuronal population with a millisecond-timescale precision in a dynamic and reversible manner . First tested in zebrafish to trigger escape responses by photo-activating Rohon-Beard neurons , ChR2 has subsequently been used to investigate diverse behaviors such as the optokinetic response or odor responses modulation . Synthetic excitatory actuators, obtained by combining a chemical ligand to a ionic channel, such as the light-gated ionotropic glutamate receptor (LiGluR, ) and the light-gated metabotropic glutamate receptor (LimGluR2, ) have been successfully used to trigger neural activity in zebrafish. For instance, the potential role of Kolmer-Agduhr interneurons in modulating slow locomotion could be investigated by combining LiGluR activation and Gal4/UAS enhancer-trap transgenics .
Optogenetics have also been used to selectively silence genetically targeted neurons in zebrafish, using the light-gated chloride pump halorhodopsin (NpHR), derived from the archaebacterium Natronomonas pharaonis ( Fig. 1 B2). NpHR hyperpolarizes neurons by pumping chloride ions upon activation with yellow light, leading to optical silencing. Interestingly, optical silencing with NpHR, and its improved variant eNpHR , can be combined with photo-activation using ChR2 to provide a versatile optogenetic toolbox to dissect circuits within the same animal .
Such combined strategy has been successfully used in zebrafish to identify neurons in the hindbrain able to initiate locomotion through a rebound activity after eNpHR silencing , or dissecting the mechanism of eye saccades during optokinetic response . In those two studies, light was delivered using optic fibers to achieve a high spatial selectivity regarding the stimulated area. However, new microscopic techniques relying on light patterning with multi-mirror devices or temporal focusing of two-photon excitation should allow for more complex 2D stimulation patterns. Lastly, 3D optical stimulation with a high spatiotemporal resolution could be achieved by combining digital holography and temporal focusing , opening the way for simultaneous imaging and neural manipulation in multiple planes in vivo .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








