In this chapter, the composition and appearance of normal muscle will be discussed. The first part will be concerned with the anatomical constituents of normal muscle at the light microscope level, followed by histochemical aspects of the different types of muscle fibres and ultrastructural details of muscle. We then discuss myogenesis and the development of muscle.
Histological Structure
The word muscle is derived from the Latin mus (= mouse) and refers to the resemblance of the muscle belly to a mouse. Muscles vary in size, shape and form, according to their function. For example, the biceps is a fusiform muscle in which the fibres are all arranged in parallel for quick activity. The deltoid is a penniform, feather-shaped muscle with a septum at an angle to the line of action which allows for maximum strength. Each muscle is enclosed in a connective tissue sheath, the epimysium, composed of extracellular matrix proteins including several collagen types and fibronectin, and merging at either end with a tendon, an aponeurosis or the periosteum of bone. Extensions of extracellular matrix from the epimysium subdivide the muscle into individual bundles or fascicles, each surrounded by a well-defined layer, the perimysium ( Figs. 3.1 and 3.2 ). The width of the perimysium varies with age and is relatively wider in neonates than in infants and adults. The diameter of individual muscle fibres that constitute the muscle bundles increases with age and with the muscle. Data from unequivocally normal muscle are ethically difficult to obtain but approximate ranges are:
- •
birth to 3 years 15–20 μm
- •
3–10 years 20–40 μm
- •
adolescent/adult females 40–70 μm
- •
adolescent/adult males 40–80 μm.


The length of fibres may reach up to 10 cm. They are closely packed to each other and in transverse section are polygonal in shape. In some muscles the fibres stretch from tendon to tendon; they also insert into the perimysial fascia. The endomysium, a network of fine collagen fibres and other extracellular matrix proteins, separates the fibres from each other. Although barely visible under normal circumstances, this extracellular matrix may proliferate in pathological muscles and become very striking.
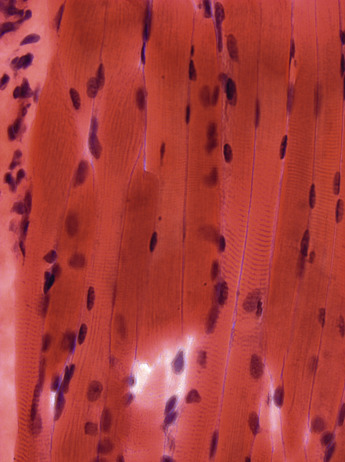
Individual muscle fibres, formed by the fusion of single cells, are multinucleated syncytia surrounded by a plasma membrane and basal lamina, the sarcolemma. The nuclei are elliptical in shape in longitudinal section and have dense peripheral heterochromatin and may have a prominent nucleolus and finely stippled nucleoplasm ( Figs. 3.3 and 3.4 ), although this is less easy to see in unfixed, frozen sections. In normal muscle most nuclei are located under the sarcolemma and in transverse section several per fibre may be visible. Nuclei of muscle fibres, except those of satellite cells (see below), do not divide. They stain blue with haematoxylin and red with the Gomori trichrome. With both these stains, variability in intensity of the fibres can be seen that correlates with differences between fibre types ( Fig. 3.5 ; see below). The mitochondria appear as small dots, red with the trichrome stain and blue with Harris’ haematoxylin.


The muscle fibre is composed of many myofibrils separated from each other by the intermyofibrillar space. In longitudinal sections it is often possible to observe the striation pattern of the myofibrils (see Fig. 3.4 ). With the use of various histochemical reactions, it is possible to distinguish the individual myofibrils, whereas the intermyofibrillar space appears as a continuous network (see below). Within the intermyofibrillar space there are various subcellular constituents, which are readily recognized under higher magnification with electron microscopy.
Various other structures can be recognized in a muscle biopsy at the light microscope level.
Blood Vessels
The vascular supply of muscle is readily apparent on routine stains and with stains such as periodic acid-Schiff (PAS), especially after diastase digestion. Medium-sized arterioles and veins run between the fascicles (see Fig. 3.1 ), while within the fascicles there is a capillary network in close relation to individual fibres (see Figs. 3.3 and 3.5 ). Type 1 fibres have more capillaries than type 2 adjacent to them, and a smaller network is apparent in muscle from neonates. The extracellular matrix components around blood vessels can be distinguished by immunohistochemistry using various antibodies (see Ch. 6 ).
Nerves
Nerves ( Fig. 3.6 ) can be demonstrated between and within muscle bundles, but they are not seen in all biopsies. The junctions of the nerves with the fibre, the neuromuscular junctions, are not easily visible with routine histological stains but can be demonstrated with certain histochemical stains, with antibodies (see Ch. 6 ), and fluorescently labelled bungarotoxin, a snake venom that specifically binds to the acetylcholine receptors. With the Verhoeff–van Gieson stain, the individual axons and the myelin sheath stain black and can be readily visualized, and with the Gomori trichrome stain the myelin of individual axons stains red ( Fig. 3.6b ). The perineurium encasing the axons is also clearly seen. Each fibre is innervated by only one nerve, although fibres are polyinnervated at early human embryonic stages. In most muscles the motor end plates form a band across the mid-belly of the muscle. In other species, such as rodents, polyinnervation is lost postnatally.

Spindles and Pacinian Corpuscles
Spindles are specialized structures consisting of striated, intrafusal fibres within a fibrous connective tissue capsule ( Fig. 3.7a ). The number of intrafusal fibres varies but is usually between 4 and 16. The spindles are located between the muscle fascicles in the perimysial connective tissue, usually adjacent to nerves or blood vessels. The fibres of spindles have their own specialized motor nerve supply (the γ efferent fibres) as well as sensory nerves. The muscle spindle acts as a sensory organ and is associated with the coordination of muscle activity and stretch, and the maintenance of muscle tone. They are found in all muscles except those of the face. For details of the physiology and anatomy of the muscle spindle, see and ( ).

Muscle fibres within the spindles are of two kinds: nuclear bag fibres, with a large collection of nuclei in the central area of the fibre, and the smaller nuclear chain fibres, with chains of nuclei throughout much of their length. With the various histochemical reactions, intrafusal fibres of spindles, similar to extrafusal fibres, vary in their enzyme activities, and attempts have been made to recognize fibre types among them (see below). Cardiac actin is present in all normal spindle fibres, and immature isoforms of myosin are present in some, even though the extrafusal fibres are fully mature ( ). Isoforms of other proteins associated with immaturity, such as those of phosphorylase, also occur in spindle fibres (see Ch. 17 ). It is important when assessing muscle biopsies that the intrafusal fibres of the spindles are not mistaken for abnormal extrafusal fibres.
The muscle spindles may be affected in certain circumstances, including sensory and motor denervation, some muscular dystrophies and ageing ( ). They have been noted to be abundant in a rare myopathy associated in some cases with mutations in the HRAS gene ( ).
Pacinian corpuscles are mechanoreceptors that respond to pressure and vibrations. Deforming a corpuscle generates an action potential in the sensory neuron attached to it. They are approximately 1 mm in length and surrounded by several concentric lamellae of connective tissue, derived from modified Schwann cells ( Fig. 3.7b ). They are named after the Italian anatomist Filippo Pacini, who described them.
Myotendinous Junction
Occasionally, a myotendinous area may be encountered in a biopsy. This is a folded zone of the outer fibre surface where the fibre tapers towards the tendon and the epimysial fascia. Sometimes, myotendinous insertions are seen in perimysial areas. Finger-like projections interleave with collagenous projections and subdivide the fibre, increasing the surface area and giving the impression of fibre size variation. Internal nuclei are common at these sites, and sometimes rod-like structures are visible ( Fig. 3.8 ). A number of proteins appear abundant at myotendinous junctions, including vinculin, talin, tenascin C, dystrophin, utrophin, laminins and certain integrins (see Ch. 6 ). Some of this enhancement may be due to the folds of the sarcolemma. Acetylcholine receptors are also present at myotendinous junctions but the reason is unknown.

Muscle Fibre Types
The application of enzyme histochemical techniques has had a major impact on the interpretation of muscle biopsies. Most skeletal muscles in humans are composed of a mixture of fibres that differ in their physiological and biochemical properties. A major aspect of muscle pathology is concerned with the identification of the fibre types and the way in which these are affected by various pathological processes. Early workers distinguished muscles by their colour, red and white. Fibres and muscles appear red because of a higher myoglobin. In animal and avian muscle this distinction can be clearly seen. For example, in chicken muscle the pectoral muscle is white compared with the darker red muscles of the thigh. Physiologists tried to characterize this colour difference on the basis of contraction: slow versus fast (see Table 3.1 ). With the advent of histochemistry it became possible to localize enzyme systems and other chemical constituents at the cellular level. This opened the way for a direct correlation of the functional activity of individual fibres with their morphology.
| Type 1 | Type 2A | Type 2B | Type 2C | |
|---|---|---|---|---|
| Colour | Red | White | ||
| Twitch speed | Slow | Fast | Fast | |
| Fatigability | Resistant | Resistant | Sensitive | |
| Twitch + oxidative and glycolytic capacity | SO | FOG | FG | |
| ATPase pH 9.4 | + | +++ | +++ | +++ |
| ATPase pH 4.6 | +++ | − | ++ | +++ |
| ATPase pH 4.3 | +++ | − | − | ++ or +++ |
| NADH-TR | +++ | ++ | + | ++ or +++ |
| Cytochrome c oxidase | +++ | ++ | + | + |
| Succinate dehydrogenase | +++ | ++ | + | ++ |
| Phosphorylase | − or + | +++ | +++ | +++ |
| PAS | + or ++ | +++ | ++ | ++ |
| Lipid droplets | +++ | ++ or +++ | + | |
| Antibodies to fast myosin heavy chain | − | +++ | +++ | ++ or +++ |
| Antibodies to slow myosin heavy chain | +++ | − | − | −, + or ++ |
Enzyme histochemistry identifies two main fibre types and a reciprocal relationship between glycolytic and oxidative enzyme activity in individual muscle fibres ( ). Type 1 fibres have high oxidative and low glycolytic activity, and type 2 fibres have low oxidative and high glycolytic activity, although there is a subtype of type 2 fibres that has a moderate oxidative capacity (see below). The nomenclature for fibre types is based on the appearance following staining for adenosine triphosphatase (ATPase), with and without preincubation at acid pH ( ). Three fibre types can be identified in normal muscle (type 1, 2A, 2B), with an additional subtype of 2C that is an immature fibre type or sometimes a hybrid fibre expressing more than one isoform of myosin ( Fig. 3.9 ).

The concept of the motor unit is fundamental to the understanding of fibre types and is important in the interpretation of pathology. The nerves innervating muscle fibres have their origin in the cell body in the anterior horn of the spinal cord. The neuron from the cell body branches to supply a variable number of muscle fibres, which in most muscles is several hundred. The anterior horn, its axon and the muscle fibres supplied constitute the motor unit, all of which are functionally dependent on each other. Muscle fibres of one motor unit are of a uniform type, and, although confined to a limited area, they are randomly scattered and not clustered. Motor units are classified by their speed of contraction and resistance to fatigue ( ). Physiologists have identified three main types: FF (fast twitch, fatigue sensitive), FR (fast twitch, fatigue resistant) and S (slow twitch, fatigue resistant). Fatigue resistance correlates with oxidative capacity and mitochondrial content, and fibres have therefore also been classified as slow twitch, oxidative (SO) that correspond to histochemical type 1 fibres, fast twitch, glycolytic (FG) that correspond to 2B and fast twitch, oxidative glycolytic (FOG) that correspond to 2A ( ). This classification was based on studies of animal muscle, but evidence suggests that human muscle is similar. Most muscles in humans, in contrast to other species, are of mixed type and show a checkerboard pattern of light and dark fibres with ATPase staining. The proportion of each fibre type, however, varies considerably between muscles and even in different regions of the same muscle ( ). Knowledge of the site from which a biopsy has been taken is therefore important when assessing the proportion of each fibre type. The tibialis anterior, for example, has a higher proportion of type 1 fibres than muscles of the quadriceps. The different enzyme profiles of each fibre type are accompanied by a multitude of fibre type-specific isoforms of structural proteins. In particular, the myosin heavy chain isoforms have been used to classify fibre types and antibodies to specific isoforms are having an increasing role in muscle pathology ( ). Four main isoforms have been identified in mammalian skeletal muscle – slow, fast 2A, fast 2B and fast 2X (also referred to as 2D) – but humans and some other large mammals such as dogs and horses do not express myosin 2B myosin protein, although they have the gene. 2B myosin RNA has, however, been reported in fetal muscle and in patients with Duchene muscular dystrophy ( ). Facial and ocular muscles express unique isoforms in addition to those that are only found during the development of other muscles. Most fibres in normal mature muscle express only one heavy chain isoform ( Fig. 3.10 ) but co-expression of more than one isoform can occur in hybrid fibres ( ). This occurs in normal muscle (e.g. 1/2A, 2A/2X hybrids) and frequently occurs in pathological muscle where it is an important aspect to assess. This is an advantage of immunohistochemistry compared with the histochemical methods for ATPase which cannot detect this co-expression. This aspect is discussed further in Chapter 6 .

However, confusion in nomenclature has arisen because of this co-expression and also because myosin isoforms have a similar Arabic letter suffix to that based on the use of ATPase. The two, however, are not equivalent. In general, type 1 fibres have slow myosin but human fibres do not express 2B myosin ( ). Thus, an ATPase 2B fibre in human muscle does not have fast 2B myosin but predominantly 2X myosin or are hybrid fibres. There is only one antibody marketed that is said to be specific to human fast 2X myosin, but fibres expressing only 2X can also be identified by exclusion of antibodies. The histochemical equivalents in human muscle have not been fully elucidated. Confusion in nomenclature also arises with fibres in pathological muscle that co-express both fast and slow myosin isoforms or immature myosin isoforms, as these may not give a clear distinction with ATPase.
Many laboratories still use the ATPase method as a standard technique for classifying fibre types, and there is a wealth of information on pathological samples based on it. With the increasing use of immunohistochemistry, however, the use of myosin antibodies is increasing. Their importance will be stressed in later chapters that discuss immunohistochemistry in detail. The advantages of using myosin antibodies are:
- •
hybrid fibres with more than one isoform can be identified and this co-expression can make clear differentiation of fibre types with ATPase difficult to achieve, particularly without acid preincubation;
- •
immature and regenerating fibres are easily identified with the appropriate myosin antibodies, and different patterns of fibres expressing immature isoforms of myosin can be seen in different neuromuscular disorders;
- •
fibre typing in postmortem muscle can be assessed with antibodies, whereas ATPase activity may be lost.
The equivalent of the histochemical 2B fibre with intermediate staining for ATPase at pH 4.6 is difficult to identify with myosin antibodies as they are often 2X or hybrid fibres, but the identification of 2B fibres is now of limited diagnostic value. In practice, the most important distinction is that between type 1 and all type 2 fibres (slow versus fast myosin fibres). In addition, 2B fibres can be identified with staining for oxidative enzyme (see below). A comparison of nomenclature and fibre type properties is given in Table 3.1 . Ultrastructural differences in fibre types are discussed below.
Plasticity of Fibre Types
Many factors can influence fibre typing, such as innervation, hormones, exercise, disuse, drugs, age and neuromuscular disorders ( ).
Early experiments on cross-innervation demonstrated the pivotal role of innervation in controlling twitch characteristics of muscle ( ). In particular, the speed at which the impulse passes down the nerve was shown to be crucial and that altering the innervation could change the histochemical and biochemical profile of a fibre ( ). This effect is known to involve calcium pathways and calcineurin and nuclear factor of activated T cells (NFAT) signalling ( ). Several different physiological and pathological factors may influence fibre types. For example, spinal cord injury induces a predominantly fast fibre profile. In myopathic disorders, however, slow myosin often predominates and fibres are consequently more fatigue resistant. Testosterone and thyroid hormones have a profound influence on fibre typing in normal and pathological situations. Hypothyroidism tends to cause a shift from fast to slow myosin, while hyperthyroidism has the opposite effect ( ). Endurance exercise training programmes increase oxidative enzyme capacity and can also influence myosin heavy chains ( ). The effects of training on muscle have led to the development of sport science as a discipline. It has been known for many years that endurance athletes have a predominance of slow/type 1 fibres, whereas sprinters have a predominance of fast/type 2 fibres. Different exercise protocols have also been developed for therapeutic and athletic benefit ( ).
Histochemical Identification of Muscle Fibre Types
This section discusses in further detail the histochemical profile of normal muscle, as it appears, with the reactions most commonly used for the assessment of pathological samples. There may be some inevitable repetition of material discussed previously, but it was felt it would be more useful to include it here rather than to refer back to previous sections.
Adenosine Triphosphatase Reactions
The reaction for ATPase is carried out at a pH of 9.4 (see Ch. 2 ), although minor adjustments may have to be made to achieve optimal results. Under these conditions, the reaction develops in the myofibrils; the intermyofibrillar network seems to dissolve out of the tissue section at some stage during the reaction. Thus, In examining an individual fibre, the myofibrils can be seen separated by an unstained intermyofibrillar network. In longitudinal section, the stain develops in the region of the bands occupied by myosin (A band). The reaction has therefore been termed the ‘myosin ATPase’ reaction.
Examining the muscle as a whole, there is a clear differentiation into two fibre types. The type 1 fibres are more lightly stained and the type 2 fibres more heavily stained at pH 9.4. Intermediate fibres are usually not seen at this pH. Following preincubation at pH 4.3, the reverse pattern is seen, with the type 1 fibres stained darkly and the type 2 fibres lightly. This reciprocal pattern is useful as dark areas are more noticeable to the eye than light. Occasionally, type 2 fibres may still retain reactivity at pH 4.3; these are the type 2C fibres. They are rare in normal human muscle but are present in developing muscle and may appear under pathological circumstances. Basophilic, regenerating fibres are usually 2C fibres. Hybrid fibres expressing fast and slow myosin isoforms may also stain as 2C fibres. Following preincubation at pH 4.6, the type 1 fibres are strongly reactive, as at pH 4.3, but the type 2 fibres can be subdivided. Some will be inhibited and stain lightly (2A), whereas others stain with an intermediate intensity (2B), giving a three-fibre pattern. 2C fibres at this pH also stain darkly (see Fig. 3.9 and Table 3.1 ). With the acid preincubation ATPase reactions, the intermyofibrillar network pattern is well demonstrated, as well as the myofibrils. The intermyofibrillar network can be removed by preincubation with calcium, which will not affect the relative staining characteristics of the various fibre types.
If the pH is increased up to 10, a three-fibre pattern can also be obtained. The precise pH for preincubation required to give a good differentiation pattern may have to be determined empirically, and conditions for human muscle are not suitable for all species. As discussed previously, the subdivision of type 2 fibres is often, but not always, of limited diagnostic value, and the most important distinction is between type 1 and type 2 fibres. A detailed description is included here, however, as the technique is performed and still favoured in many laboratories and it is relevant to the interpretation of several stains. Immunolabelling of myosin isoforms is discussed in Chapter 6 and is the technique we have used exclusively in London for many years.
Oxidative Enzymes
The various oxidative enzyme reactions show some similarity in the appearance of the sections. Therefore, they will be discussed together and the minor variations will be pointed out.
Reduced Nicotinamide Adenine Dinucleotide-Tetrazolium Reductase
With this reaction, two fibre types can be recognized in normal muscle, or three types (types 1, 2A and 2B) if the section is not over-incubated or the concentration of substrate not too high ( Fig. 3.11 ). Type 1 fibres show a darker blue colour than the type 2 fibres, and type 2A fibres are of intermediate intensity. The myofibrils are unstained, but the intermyofibrillar network, comprising the mitochondria and sarcoplasmic reticulum, is well demonstrated ( Fig. 3.11b ). This network pattern is slightly different in the two fibre types. Correlation of the reduced nicotinamide adenine dinucleotide-tetrazolium reductase (NADH-TR) reaction with ATPase activity of fibres on serial section shows that, in human muscle, type 1 fibres (weak with ATPase at pH 9.4) react most intensely to NADH-TR, type 2B fibres show the least reaction with NADH-TR, and the type 2A fibres have an intermediate activity (see Table 3.1 ).











