Evidence of nonopioid analgesic effectiveness exceeds that for long-term opioids in chronic noncancer pain (CNCP), most with lower risk. Non-drug therapies such as cognitive behavioral therapy and physical activation are safer and also effective. Nonsteroidal antiinflammatory drugs are useful for inflammatory and nociceptive pain, share renal and variable gastrointestinal, bleeding and cardiovascular side effects. Antidepressants with noradrenergic activity (such as tricyclics and seroton-norepinephrine reuptake inhibitors) and neuromodulating anticonvulsant drugs (gabapentinoids and sodium-channel blockers) are proven to be effective for neuropathic and centralized pain. Ketamine and cannabinoids are other studied analgesics but have a less well-proven role in CNCP.
Key points
- •
Although there is evidence supporting the analgesic efficacy of opioids, other classes of medications seem to be equally effective, and are often safer to use.
- •
Nonsteroidal antiinflammatory drugs are most likely to be effective in the setting of nociceptive or inflammatory pain.
- •
Antidepressants and anticonvulsants are the most effective agents for neuropathic pain or pain from central sensitization.
- •
Unless there is clear evidence of spasticity, muscle relaxers, including benzodiazepines, and antispasticity agents add limited, if any, benefits in the management of chronic pain.
- •
Benzodiazepines should not be used in conjunction with opioids.
- •
An important early target for pharmacologic management should be improvement in the patient’s ability to sleep.
Introduction
Treatment of pain, particularly chronic pain, requires multimodal analgesia that includes pharmacologic and nondrug therapies as well as a large measure of clinician-guided patient self-management. This article emphasizes the point that pain medicine is more than opioid management; that chronic pain treatment should rarely if ever be opioid monotherapy. The conflation of pain care with opioid prescribing has, since the mid 1990s, displaced the initial tenets of multidisciplinary pain care that were fundamentally about functional and behavioral retraining without opioids and other central nervous system depressants. Recent population-based trends document that significant increases in opioid prescribing without increases in other drug categories have directly contributed to the pain-related distress experienced not only by patients with chronic pain but by providers of pain care, and health systems. Understanding the basic pharmacologic principles and comparative effectiveness of so-called adjuvant drugs in the management of chronic pain should increase not only success of pharmacologically based pain treatments but also reduce over-reliance on opioids and the associated challenges of opioid-related side effects such as accidental overdoses, dependency and addiction, and most importantly poor pain care outcomes.
Principles and Definitions
A key principle guides this article: chronic pain is a complex multidimensional disorder that is different from acute pain in both pathoanatomic and psychosocial domains. Although many commonly encountered chronic pain disorders do involve continuing nociceptive triggers (nociceptive pain results when tissue injury or disease leads to stimulation of nociceptors at peripheral sensory nerve endings located in the skin, muscle, joints, and viscera), the persistence of pain beyond 3 to 6 months alters peripheral and central nervous function and, with additional behavioral and functional modifications, adds the insult to the original injury. This pathophysiologic process involves sensitization, whereby the intrinsic neuroplasticity of the nervous system disrupts previously normal (or worsens previously abnormal) neurochemical and structural function of the peripheral and central nervous system, altering neuronal activity, their synapses, and the brain’s regional connectivity. Current functional imaging data support the emerging understanding that, over time, nearly all chronic nociceptive stimulation, especially persistent and repetitive high input, becomes transformed in a process called chronification. In addition, susceptibility to chronification varies by complex mechanisms determined by an increasingly well-understood overlay of genetic predisposition, epigenetic biological processes, psychological responses, and sociologic exposures. Hence pain, an “unpleasant sensory and emotional experience that is commonly associated with actual or potential tissue damage,” is an emergent experience, shaped by the complex interplay of genetics, past experiences, setting, affect, cognitive context, and cultural and social expectations. Patients with both acute and chronic pain thus present with a wide range of responses to seemingly identical injuries, and effective treatment requires a clinical toolbox capable of multidimensional therapies.
Assessing Effectiveness
Consideration of effectiveness is essential to clinicians’ proper selection of pain treatments. An expanded appreciation and more detailed understanding of the complex nature of pain entail multimodal assessment of pain beyond a simple numeric pain intensity score (numerical rating scale [NRS]) of 0 to 10 out of 10. Although pain rating is useful in acute pain, in which reduction in pain intensity can be achieved quickly and effectively with opioids, nerve blocks, and general anesthetics, successful chronic pain care requires demonstration of improved measures of physical, emotional, and social function, and thus overall quality of life. Hence, this article references treatment goals beyond how much it hurts; successful pain management requires careful tracking of outcomes across key domains of physical (including sleep), psychological, and social function.
High-quality multidisciplinary pain care also reduces overall health care costs by adding system value. Literature since the 1970s has supported the positive effects of multidisciplinary pain care on function, mood, and quality of life. Because health care delivery is obliged to move toward value-based care, in which value equals quality of patient satisfaction, family experience, and improved psychosocial outcomes at lower costs, clinically useful knowledge of alternatives to opioids is needed.
Introduction
Treatment of pain, particularly chronic pain, requires multimodal analgesia that includes pharmacologic and nondrug therapies as well as a large measure of clinician-guided patient self-management. This article emphasizes the point that pain medicine is more than opioid management; that chronic pain treatment should rarely if ever be opioid monotherapy. The conflation of pain care with opioid prescribing has, since the mid 1990s, displaced the initial tenets of multidisciplinary pain care that were fundamentally about functional and behavioral retraining without opioids and other central nervous system depressants. Recent population-based trends document that significant increases in opioid prescribing without increases in other drug categories have directly contributed to the pain-related distress experienced not only by patients with chronic pain but by providers of pain care, and health systems. Understanding the basic pharmacologic principles and comparative effectiveness of so-called adjuvant drugs in the management of chronic pain should increase not only success of pharmacologically based pain treatments but also reduce over-reliance on opioids and the associated challenges of opioid-related side effects such as accidental overdoses, dependency and addiction, and most importantly poor pain care outcomes.
Principles and Definitions
A key principle guides this article: chronic pain is a complex multidimensional disorder that is different from acute pain in both pathoanatomic and psychosocial domains. Although many commonly encountered chronic pain disorders do involve continuing nociceptive triggers (nociceptive pain results when tissue injury or disease leads to stimulation of nociceptors at peripheral sensory nerve endings located in the skin, muscle, joints, and viscera), the persistence of pain beyond 3 to 6 months alters peripheral and central nervous function and, with additional behavioral and functional modifications, adds the insult to the original injury. This pathophysiologic process involves sensitization, whereby the intrinsic neuroplasticity of the nervous system disrupts previously normal (or worsens previously abnormal) neurochemical and structural function of the peripheral and central nervous system, altering neuronal activity, their synapses, and the brain’s regional connectivity. Current functional imaging data support the emerging understanding that, over time, nearly all chronic nociceptive stimulation, especially persistent and repetitive high input, becomes transformed in a process called chronification. In addition, susceptibility to chronification varies by complex mechanisms determined by an increasingly well-understood overlay of genetic predisposition, epigenetic biological processes, psychological responses, and sociologic exposures. Hence pain, an “unpleasant sensory and emotional experience that is commonly associated with actual or potential tissue damage,” is an emergent experience, shaped by the complex interplay of genetics, past experiences, setting, affect, cognitive context, and cultural and social expectations. Patients with both acute and chronic pain thus present with a wide range of responses to seemingly identical injuries, and effective treatment requires a clinical toolbox capable of multidimensional therapies.
Assessing Effectiveness
Consideration of effectiveness is essential to clinicians’ proper selection of pain treatments. An expanded appreciation and more detailed understanding of the complex nature of pain entail multimodal assessment of pain beyond a simple numeric pain intensity score (numerical rating scale [NRS]) of 0 to 10 out of 10. Although pain rating is useful in acute pain, in which reduction in pain intensity can be achieved quickly and effectively with opioids, nerve blocks, and general anesthetics, successful chronic pain care requires demonstration of improved measures of physical, emotional, and social function, and thus overall quality of life. Hence, this article references treatment goals beyond how much it hurts; successful pain management requires careful tracking of outcomes across key domains of physical (including sleep), psychological, and social function.
High-quality multidisciplinary pain care also reduces overall health care costs by adding system value. Literature since the 1970s has supported the positive effects of multidisciplinary pain care on function, mood, and quality of life. Because health care delivery is obliged to move toward value-based care, in which value equals quality of patient satisfaction, family experience, and improved psychosocial outcomes at lower costs, clinically useful knowledge of alternatives to opioids is needed.
Article objectives
- 1.
Understand why chronic pain pharmacologic management should not be opioid monotherapy.
- 2.
Identify the clinical value and potential side effects of nonsteroidal antiinflammatory drugs (NSAIDs), antidepressants, anticonvulsants, and other pain neuromodulators.
- 3.
Optimize use of nonopioid drug options for management of chronic pain.
Why nonopioids?
The preceding discussion underpins the consensus recommendation from pain experts: “opioids are (just) part of the plan…” A basic limitation of opioid monotherapy is that, at doses needed to maintain conscious awareness (that is, less than anesthetic levels), the effectiveness of opioids in chronic pain has not been clearly shown. A recent evidence-based literature review of long-term opioid effectiveness by Chou and colleagues concluded that the best available evidence is low quality, and most is insufficient. In the context of the current epidemic of prescription opioid overdoses, deaths (>16,000 in the United States in 2012), and parallel increase in addiction treatment, expertise in nonopioid pain pharmacotherapy is essential. Complications of increased falls, risks with co-occurring medical conditions (especially sleep apnea), and hypogonadism are salient. The recommendation by some pain specialists during the past 20 years that reductions in long-term analgesic effectiveness of opioids (typically because of tolerance) can be overcome by routinely increasing the opioid dose is no longer seen as appropriate, particularly because there is emerging clinical and scientific evidence of opioid-induced hyperalgesia.
Best evidence currently supports multimodal analgesia, which is the use of combinations of analgesic medications with different mechanisms of action. The classes of medications that should be considered are:
- •
Opioids
- •
NSAIDs
- •
Local anesthetics
- •
Antiepileptics
- •
Antidepressants
- •
N -methyl- d -aspartate (NMDA) antagonists
- •
Cannabinoids
Even when efficacy is limited to just assessment of numeric pain intensity, and acknowledging that the quality of evidence is limited because of problems with study duration, nonrepresentative patient cohorts, and small effect sizes, many nonopioids have effectiveness at least as good or better than opioids in chronic noncancer pain (CNCP) ( Table 1 ).
| Opioids (%) | 30 |
| Antidepressants a (%) | 30 |
| Anticonvulsants (%) | 30 |
| Acupuncture (%) | 10 + |
| Cannabis (%) | 10–30 |
| CBT/mindfulness (%) | 30–50 |
| Physical fitness (%) | 7–50 |
| Sleep restoration (%) | 40 |
| Hypnosis, manipulations, yoga | Some effect |
a Antidepressants include tricyclics and serotonin-norepinephrine reuptake inhibitors.
Although the scope of this article does not include extended reviews of multimodal nondrug analgesia, these are included for completeness ( Box 1 ). Goals include improved patient self-management by reduced catastrophizing, fear avoidance, and pain anticipation. Nondrug treatments are promoted by deemphasizing medications as a central element for managing pain, empathic listening interwoven with respectful response, sharing development of mutually agreed-on care goals, and self-identification of strategies and support needed to reduce medication-seeking behaviors. Outcomes include an increased ability to tolerate pain to fulfill life roles for family and job to increase overall quality of life. Effective use of these techniques requires a well-integrated team of interprofessionals, including but not limited to physical, occupational, and behavioral therapists and often nurse care educators.
- •
Cognitive: identify distressing negative cognitions and beliefs
- •
Behavioral approaches: mindfulness, relaxation, biofeedback
- •
Physical: activity coaching, graded exercise (land and aquatic) with physical training, class, trainer, and/or solo
- •
Spiritual: identify and seek meaningfulness and purpose of life
- •
Education (patient and family): promote patient efforts to increase functional capabilities
Diagnosis-based selection of pharmacotherapy
Medical treatment decision making requires a careful, detailed, systematic, structured history and physical examination. Careful empathic listening permits patients to communicate their pain narratives and attributions, and to disclose many domains of relevant history. The history as obtained by interview should be supplemented with evidence-based tools that assess mood and psychiatric diagnoses (ie, personal health questionnaire, 9-item [PHQ-9], generalized anxiety disorder, 7-item [GAD-7], posttraumatic stress disorder [PTSD] screening ), sleep (quality and duration), important co-occurring conditions such as sleep apnea (snore, tired, obstructed breathing, high blood pressure, body mass index, age, neck size, gender [STOP-BANG] ), and risk of drug misuse and addiction (ie, opioid risk tool [ORT], screener and opioid assessment for patients with pain-revised [SOAPP-R], diagnosis, intractability, risk, efficacy [DIRE]). Use of measurement-based tools for assessment of chronic pain is described in detail by this author in the January 2013 IASP Clinical Update . Once diagnoses are established across biopsychosocial domains, drug treatments can be formulated based on type of pain (such as nociceptive and/or neuropathic), behavioral diagnoses, and risk assessments (summarized in Box 2 ).
- •
Nociceptive pain
- ○
Rest, ice, NSAIDs, opioids
- ○
- •
Neuropathic pain
- ○
Tricyclic antidepressants (TCAs), serotonin-norepinephrine reuptake inhibitor (SNRIs), antiepileptics, opioids
- ○
- •
Co-occurring pain syndromes (functional or central sensitization)
- ○
TCAs, SNRIs, antiepileptics
- ○
- •
Depression and anxiety
- ○
Antidepressants, cognitive behavior therapy (CBT)/counseling
- ○
- •
PTSD
- ○
Prazosin, antidepressants, CBT/dialectical behavioral therapy (DBT)
- ○
- •
Opioid use disorder
- ○
Substance abuse treatment:
- ○
Abstinence or medication-assisted treatments (ie, methadone maintenance or Suboxone)
- ○
Improving Sleep Equals Reducing Pain
Sleep management is a key first step to reducing pain intensity and improving quality of life in patients with both acute and chronic pain. Restorative sleep has been reported to predict the resolution of chronic widespread pain. Although sleep treatment is not typically considered analgesic, research has found that sleep loss, and in particular rapid eye movement (REM) sleep loss, are acutely hyperalgesic. For example, compared with insomniacs, individuals with normal sleep patterns rate experimental pain stimuli as 50% less intense, and have pain thresholds that are 50% higher. An important first step to effective pain management is to develop a sleep management program that achieves the patient sleeping at least 6 hours at night. Morin and Benca published an excellent review of insomnia management in Lancet 2012.
Nonpharmacologic options typically start with review of so-called sleep hygiene techniques ( Box 3 ). Additional supplemental strategies are often needed to support sleep hygiene, such as sleep restriction therapy, which improves sleep efficiency by limiting sleep time to initially conform to the patient’s own recorded sleep log; once efficiency is restored, sleep time can then return to 7 to 8 hours nightly. Cognitive behavior therapy for insomnia is a well studied and effective nondrug strategy for insomnia.
- •
Maintain a regular wake/sleep schedule: fixed bed and wake-up times, regardless of weekday or weekend
- •
Refrain from taking naps
- •
No caffeine after noon, if at all
- •
Exercise, but not within 3 hours of bedtime
- •
Establish a relaxing routine before bedtime
- •
No exposure to television or computer screens 2 hours before bedtime
- •
Use the bedroom for sleep activities
- •
Set environment (light, noise, temperature) at comfortable levels
- •
Avoid alcohol close to bedtime
Patients with sleep difficulties typically have already tried nonprescription melatonin (1–5 mg), which triggers the sleep circadian cycle, but often take it just before bed, whereas sleep experts recommend that melatonin is more helpful when taken up to 6 hours before bedtime. Patients commonly have tried OTC remedies that include antihistaminic agents; although sedating, antihistamines are not analgesics, even in the setting of interstitial cystitis, for which they are often prescribed. There is limited evidence that diphenhydramine improves insomnia, and routine use is generally not recommended.
Tricyclic antidepressants (TCAs) in general decrease sleep latency, increase total sleep time, but decrease REM sleep duration. Gabapentinoids increase REM, total sleep time, and slow wave sleep. Although they have not been systematically studied for sleep, clinical experience suggests that they are often useful sleep drugs. Both TCAs and gabapentinoids are sedating and assist sleep initiation and maintenance via different mechanisms: TCAs via antihistaminic side effects, and gabapentinoids by modulating the release of excitatory neurotransmitters. TCAs may be very effective sleep aids, especially those with higher antihistaminic properties, like doxepin (specifically approved for insomnia) and others such as amitriptyline, nortriptyline, and imipramine. Serotonin-norepinephrine reuptake inhibitors (SNRIs) are less antihistaminic, so direct sedative benefits are not expected; they may also disturb sleep by provoking periodic leg movement disorders. Trazodone promotes sleep via 5-hydroxytryptamine (5-HT) 2A antagonism, but analgesic properties have not been shown; prescribing trazodone requires caution when combined with other serotonergic drugs such as selective serotonin reuptake inhibitors (SSRIs), SNRIs, triptans, and tramadol. SSRIs decrease REM sleep, increase REM latency, and fragment sleep. The antidepressant mirtazapine, especially at lower and mostly antihistaminic doses (ie, 7.5–15 mg), is an effective sedative agent, and although not evaluated as an analgesic, would be expected to be so based on its moderate noradrenergic effects. Prazosin is effective at reducing nightmares in patients with PTSD, and can be useful in patients with chronic pain and co-occurring PTSD. Although many prescribers recommend that patients use opioids for sleep, intending to reduce pain-related awakening, opioid medications are associated with dose-related central and obstructive sleep apnea, and are associated with electroencephalogram-demonstrated frequent awakenings, and reduce duration of slow wave sleep, which may be particularly restorative via antiinflammatory mechanisms.
Use of benzodiazepines for sleep is not recommended in chronic pain based on lack of evidence for sustained benefits; rebound insomnia; risk of oversedation, especially when combined with opioids; and the complicating development of tolerance, dependency, and addiction. Benzodiazepines also decrease slow wave sleep as well as REM sleep. The nonbenzodiazepine hypnotic drugs (z-drugs: zolpidem, zaleplon, eszopiclone) risk parasomniac complex behaviors such as sleep walking, sleep eating, and driving unaware; disturb EEG sleep architecture; and their use does not improve patient-reported pain scores, so are of limited value in patients with chronic pain. The Beers criteria for potentially inappropriate medication use in older adults strongly recommend against use of sedative hypnotics in older patients based on moderate quality of evidence, although clinical judgment may find exceptions in the setting of palliative and hospice care. Only a few studies have evaluated long-term effectiveness, although addiction to nonbenzodiazepines is generally considered unlikely.
Acetaminophen
Widely used, inexpensive, and available without prescription (OTC), acetaminophen (paracetamol) is an effective low-potency analgesic and antipyretic agent. Although the mechanism of effect of acetaminophen remains uncertain, its action may be on the transient receptor potential (TRP) A1 ion channel expressed on the central terminals of primary sensory neurons in the dorsal horn of the spinal cord. It has also been cited as either a cyclooxygenase (COX) 2 or 3 inhibitor (review of COX actions is presented later), and has been proposed to be more active as a central nervous system analgesic than an antiinflammatory. At doses of less than 4 g daily it is well tolerated and safe for those less than 60 years of age; current recommendations suggest doses at or less than 2 g daily for older patients. Risks of hepatotoxicity increase significantly based on dose, older age, combination with alcohol, and co-occurring liver disease (ie, hepatitis C, alcoholic cirrhosis). Acetaminophen-related hepatic failure is the leading cause of acute liver failure worldwide. Cautious medication review and reconciliation of OTC use of acetaminophen is important for all patients with pain, not only regarding its role as an analgesic but also its presence in combination with a wide range of other minor cold and influenza products. Be alert to acetaminophen in combination with many prescribed opioid analgesics, such as codeine (ie, Tylenol #3), hydrocodone (ie, Vicodin), and oxycodone (ie, Percocet).
Analgesic nephropathy (renal papillary necrosis and interstitial nephritis) is caused by chronic use of phenacetin (a prodrug of acetaminophen) in combination with aspirin, and possibly from chronic high-dose use of acetaminophen and/or NSAIDs, and probably in the setting of underlying chronic kidney disease.
Combination with NSAIDs adds analgesic efficacy, but is also associated with greater risks of gastric and renal injury than with NSAIDs alone.
- •
Effective analgesic, without antiinflammatory or coagulation effects
- •
Mechanism of action still not fully understood
- •
Caution regarding doses more than 2 g/d
- •
Be alert to OTC use for colds and fever, and/or presence in combination with other analgesics
Nonsteroidal Antiinflammatory Drugs
NSAIDs are the mostly widely used pain relievers, with 60 million US prescriptions yearly, the elderly consuming 3.6 times more than younger adults; they overall account for 25% of adverse drug reactions reported. NSAIDs are available in more than 20 formulations, via oral, intravenous (IV), and transdermal routes of administration. NSAIDs are recommended for nociceptive pain, defined as pain that arises from actual or threatened damage to nonneural tissue and is caused by the activation of nociceptors. They are commonly used for traumatic musculoskeletal pain syndromes, such as muscle, ligament, and or tendon injuries, and joint disorders from traumatic, infectious, or degenerative conditions. Good-quality evidence shows effectiveness for spinal pain from disc, facet, or spinal ligament injuries. Neuritis related to connective tissue disorders also are NSAID responsive. Rheumatic pain syndromes, such as inflammatory myositis, tendonitis, enthesopathy, and autoimmune arthritides, and spondyloarthropathies, as well as crystalline arthritides respond to NSAIDs. NSAIDs are useful in the management of endometriosis pain. NSAIDs may exacerbate preexisting inflammatory bowel disease, complicate diverticulitis, and are associated with collagenous colitis.
NSAIDs reduce pain resulting from activation and sensitization of peripheral nociceptors. Phospholipase A 2 releases arachidonic acid from membrane lipids, which then goes on to synthesize the eicosanoid autoacids, hydroperoxidase and endoperoxidase. These cyclized eicosanoids are then converted by COX-1 and COX-2 into key components of the inflammatory cascade: prostaglandins (PGs) and prostacyclins, which go on to sensitize a heterogeneous range of nociceptors, and thromboxanes, which trigger platelet aggregation and leukocyte modulation ( Fig. 1 ).
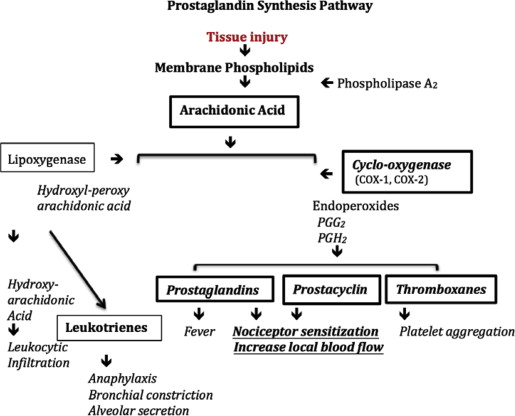
Patients with pseudoallergic NSAID asthma seem to be more susceptible to COX-1 specificity from acquired alterations in arachidonic acid to leukotriene pathways, and do not represent an immunoglobulin E–mediated allergy.
Selective Cyclooxygenase 2 Inhibition
A notable pharmacologic advance in the management of inflammatory pain followed the discovery of the distinct enzymatic categories of COX-1 and COX-2. The subsequent identification of NSAIDs with more or less selective targeting of COX-2 inhibition was heralded as a major therapeutic breakthrough, because COX-1 was thought to be a homeostatic (or constitutive) rather than reactive (or inducible) progenitor of PGs. Subsequent clinical evidence has shown that COX-2 selectivity reduces bleeding risks, because, in contrast with COX-1 agents, it does not reduce thromboxane A 2 , a compound required for platelet aggregation. Platelet covalent acetylation with aspirin or other acetylated NSAIDs prevents normal platelet aggregation for the life of the platelet, so restoration of normal coagulation requires about 10 days, which is the time needed to recover adequate numbers of nonexposed normally functioning platelets. Short-acting nonacetylated NSAIDs require discontinuation for only the half-life of the drug (usually 3–5 days) to prevent thromboxane interference with coagulation, which is an issue when considering at-risk interventional procedures.
Reduced gastric and duodenal mucosal injury side effects of selective COX-2 NSAIDs are important advantages, although incomplete. COX-1 inhibits PG I 1 , which does confer cytoprotection of gastric endothelium. However, COX-2 inhibits production of PG E 2 which also plays a significant role in protection of gastric and duodenal mucosa. Although COX-2 selectivity avoids some of the additive COX-2 effects, subclinical endoscopic gastrointestinal (GI) injury of nonselective COX-1 inhibition is only lessened by about half, reduced from between 8% and 18% (nonselective NSAIDs) to between 4% and 12% (selective COX-2). Published evidence supports the addition of any of the widely available proton pump inhibitors or H 2 blockers for patients with a prior history of gastritis or gastroesophageal reflux disease. Misoprostol (Cytotec; a synthetic PG E1 analogue) can be prescribed separately or in combination with diclofenac. Misoprostol is less well tolerated than proton pump inhibitors, because of bloating and other nonspecific abdominal complaints, is contraindicated in pregnancy, and there are concerns in women of child-bearing age because of an abortifacient effect.
Because NSAID use during first and second trimesters have been associated with a variety of fetal risks, as a class they are listed as category C; routine use during early pregnancy is best avoided. NSAIDs are contraindicated in late term pregnancy because of inhibition of closure of the ductus arteriosus, oligohydramnios, and risk of traumatic bleeding at delivery.
Nonsteroidal Antiinflammatory Drugs and Cardiovascular Disease
All NSAIDs, whether COX-2 selective or not, increase risk of cardiovascular (CV) disease, for patients with and without out preexisting risk. This increased risk is likely caused in large part by the well-established increase of 5 mm Hg in normotensive patients and more than 10 mm Hg increase in blood pressure (BP) in hypertensives resulting from PG interference, most likely in the kidney. All NSAIDs interfere, at times significantly, with BP management, countering the effects antihypertensive drugs (especially angiotensin-converting enzymes, angiotensin receptor blockers, and diuretics.) COX-2 selective agents cause prostacyclin activity reduction without COX-1 antiplatelet thromboxane benefits, possibly promoting additional endothelial injury, although celecoxib (a COX-2 selective) at 200 to 400 mg/d seems to present a risk comparable with that of naproxen (a nonselective NSAID). Acetaminophen also increases BP and CV risk. Patients at low CV risk seem to have a very small (<1%/y) dose-related increase in risk of myocardial infarction, congestive heart failure, arrhythmia, and stroke, which is so small an increase that NSAIDs are appropriate when indicated and effective. In patients with known CV disease, risk of new vascular events increases in excess of 0.7%/y from a baseline of 2%. Compared with opioids, NSAIDs seem to increase CV risk by 1.8, although non-CV risks of chronic opioids are higher. Naproxen seems not to present this same increased risk compared with diclofenac, ibuprofen, and celecoxibs, so it can be considered the safest NSAID overall.
Other Major Nonsteroidal Antiinflammatory Drug Risks
Nonsteroidal antiinflammatory drugs and the kidney
Nephrotoxicity from NSAIDs is related to inhibition of glomerular and endothelial renal vasodilation from both COX-1 and COX-2 inhibitors with net lowering of glomerular filtration, more severely so when patients are volume depleted. Risks worsen in older patients, those with preexisting kidney disease, and before procedures involving radiocontrast agents. NSAIDs also increase potassium levels in patients receiving potassium-sparing antihypertensives.
Reye syndrome
NSAIDs for the treatment of fever in children (aspirin has been shown, but circumstantially all NSAIDs) are associated with Reye syndrome. Acetaminophen management of fever in children is recommended instead.
Nonsteroidal antiinflammatory drugs, bones, and tendons
NSAIDs have a causal relationship with nonunion following bone fracture and spine fusion, although this has not been shown in humans. There is no evidence of poor tendon healing in rodents either.
Nonsteroidal Antiinflammatory Drug-Drug Interactions
NSAIDs displace protein-bound phenytoin and warfarin, increasing their activity. GI bleeding from COX-1 NSAIDs, especially in anticoagulated patients, can be severe and catastrophic. Increased renal toxicity is seen in combination with methotrexate and cyclosporine. Serum levels of lithium are significantly increased when combined with NSAIDs. Although hepatic cytochromes (2C9 and 1A2) metabolize several NSAIDs, this is not a clinically significant concern. Other side effects (especially with aspirin) include dose-related salicylism causing tinnitus and hyperventilation. The sulfonamide structure of celecoxib increases risk of allergic reaction in patients with sulfa allergies.
Transdermal Nonsteroidal Antiinflammatory Drugs
Formulations of diclofenac that are transdermally absorbed show analgesic efficacy that is much less than that of orally administered oral NSAIDs, with demonstrated plasma maximum concentration (C max ) bioavailability varying from just 0.2% to 8%. Penetration depth varies with product formulation, but is typically only 3 to 4 mm, although it can be 2-fold deeper with occlusion, so the drugs are unable to reach the synovium and/or periosteum of many joints, which are the inflammatory target sites. Hence, they are recommended for joints proximate to the skin, like phalanges or wrists. Diclofenac with the absorption enhancer dimethyl sulfoxide (Pennsaid) is approved for knee osteoarthritis. Because of low systemic absorption, adverse effects relate mostly to skin site application.
Monitoring Nonsteroidal Antiinflammatory Drugs
Monitoring recommendations with chronic use vary, although it is prudent to obtain a baseline creatinine level for anything more than very short-term use for all prescribed NSAIDs. Cautious prescribing for long-term use merits an annual creatinine level, and with any concerns regarding renal impairment consider either discontinuation or an annual or alternate-year calculation of glomerular filtration or creatinine clearance determination.
- •
Useful for nociceptive pain
- •
COX-2 selectivity reduces bleeding and GI side effects
- •
All NSAIDs increase BP and CV risks
- •
All risks increase with age and dose
- •
Monitor renal function with prolonged use
Corticosteroids
Brief mention is made of corticosteroids in pain management because they play a limited role as agents effective in acute and subacute pain related to tendon and synovial injuries and inflammatory processes, including those related to cervical or lumbar disc herniation with radiculopathy. Selective injections into joint and tendon sites may also be useful, with or without ultrasonography guidance. A short tapering course of oral prednisone (starting dose of 20–40 mg) or methylprednisolone (starting dose of 24–48 mg) may be considered in an acute radiculopathy without abnormal motor findings before beginning a more detailed imaging evaluation. Long-term use for chronic rheumatologic diseases, such as rheumatoid arthritis, lupus, spondyloarthropathies, and polymyalgia rheumatica, is best reserved for clinicians with special training in management of these chronic conditions. Toxicity includes glucose intolerance, especially in those with established diabetes, even with epidural administration. Sodium retention is a common adverse effect, even with short-term use. Exacerbation of anxiety and depression, sleep disruption, and delirium may occur. Long-term and/or high-dose use raises risks of severe adverse effects on bone mineral metabolism (ie, osteopenia and osteoporosis), and osteonecrosis has been reported with doses greater than or equal to 20 mg/d ; cautious use, adequate informed consent, and careful monitoring are strongly advised.
Antidepressants for pain
Antidepressants classified as tricyclic (TCAs) and tetracyclic, SNRI, SSRIs, norepinephrine-dopamine reuptake inhibitors, and serotonin modulating drugs are, to varying degrees monoamine (norepinephrine, serotonin, and dopamine) reuptake inhibitors and presynaptic alpha 2 -adrenergic antagonists that increase availability of norepinephrine and serotonin in the central nervous system. Antidepressant analgesia is multifactorial, because potential sites of action extend across both peripheral and central signaling and transmission systems ( Table 2 ).
| Mechanism of Action | Site of Action | TCA | SNRI | SRI |
|---|---|---|---|---|
| Reuptake inhibition of monoamine | Serotonin | + | + | + |
| Noradrenaline | + | + | − | |
| Receptor antagonism | Alpha-adrenergic | + | − | − |
| NMDA | + | (+) Milnacipran | − | |
| Blocker or activator of ion channels | Sodium channel blocker | + | (+) Venlafaxine-duloxetine | (+) Only fluoxetine |
| Calcium channel blocker | + | ? | (+) Citalopram fluoxetine | |
| Potassium channel activator | + | ? | − | |
| GABA B receptor | Increase of receptor function | + Amitriptyline desipramine | ? | + Fluoxetine |
| Opioid receptor binding/opioid-mediated effect | μ and δ opioid receptor | (+) | (+) Venlafaxine | (+) Paroxetine |
| Inflammation | Decrease of PG E 2 production | + | ? | + Fluoxetine |
| Decrease of TNFα production | + | ? | ? |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





