Neurological Injury in the Lumbar Spine
Mukund Gundanna
Jeffrey C. Wang
OVERVIEW
Lumbar spine surgery can be rewarding to both the patient and the surgeon. The thecal sac can be manipulated in such a way that from a posterior approach, it can be visualized in its entirety anteriorly, posteriorly, and laterally. The intervertebral disc can be inspected and can be debrided or removed, depending on the surgical plan. The nerve roots can be traced from their origins at the dural sleeve and can be explored as they leave through the neural foramen under the pedicle and out toward the retroperitoneal space. Often, the neural elements can be decompressed uneventfully, and the bony elements can be defined. The spine can be instrumented and bone grafted, and the neural elements can be monitored when needed. However, if dural sleeve compromise is encountered during surgery, repair and the postoperative course can be complex, depending on the location and size of the tear and the quality of the tissue. When neural injury occurs as a result of intraoperative stretch, irritation, compression, laceration, avulsion, or by instruments or implants, the process must be quickly recognized and reversed, if possible, to provide the best chance to recover from such events.
This chapter will attempt to identify, analyze, and suggest treatments for the various common mechanisms of iatrogenic neurological injury in the lumbar spine in the context of spine surgery complications. Additionally, neurological monitoring will be discussed in a context relevant to the spine surgeon.
INCIDENCE OF THE COMPLICATION
Durotomy
Incidental durotomy is not uncommon in spine surgery. In primary surgery, it can be caused by eroded or thin dura, adhesion, fibrosis, or redundancy in cases of spinal stenosis. A history of multiple injections can alert the surgeon to many of these potential problems. In revision surgery, direct dural scarring can be found in addition to the above. It may be difficult to peel off the scar from the dura in many cases (1).
Wang et al. (2) noted that 14% of a pool of patients sustained an intraoperative dural tear. Over half of those durotomies were in revision cases. Stolke et al. (3) found that 5.3% of open discectomies, 1.8% of microdiscectomies, and 17.4% of revision surgeries had a recognizable intraoperative dural tear. Cammisa et al. (4) reported an overall durotomy rate of 3.1%, with about a 2% to 3% rate in primary surgeries, and an 8.1% rate in revisions. Certainly, the total number of dural tears is much larger since many go unrecognized and usually on to a benign course. Typically, with proper treatment of dural tears, there are no significant long-term sequelae (2,4).
Complications of Durotomy
Some durotomies do not heal, instead becoming persistent leaks. These may or may not have been recognized during surgery and, of those recognized, some may not have been repairable.
In Cammisa et al.’s study (4), the 67 patients with recognized and treated intraoperative durotomies underwent relatively benign postoperative courses and had no significant long-term sequelae. In the same study, 0.28% of patients had a clinically significant postoperative leak that went unrecognized during surgery (4). Of these six patients, five had pseudomeningoceles and one had fluid egress from the surgical site. They reported varying signs and symptoms of cerebrospinal fluid (CSF) leak such as postural or persistent headache, meningeal irritation, neurological deficit,
and palpable fullness at the surgical site. The reported rate for pseudomeningocele formation after lumbar surgery has been estimated to be between 0.07% and 2% (4).
and palpable fullness at the surgical site. The reported rate for pseudomeningocele formation after lumbar surgery has been estimated to be between 0.07% and 2% (4).
In Wang et al.’s study, 88 patients had recognized intraoperative tears. None of those patients developed a myelocutaneous fistula, but two required reoperation and revision dural closure for persistent headache and signs of meningeal irritation.
Certainly, the gravest complication of persistent fluid leak is meningitis. Despite the relatively large number of durotomies, the incidence of meningitis is rare, 0.18% (5). In a large, hospital-based study, Twyman et al. reported that the most common organisms were Staphylococcus aureus, Escherichia coli, and Enterococcus faecalis (5). There are case reports in the literature of other organisms isolated, especially Acinetobacter (6). In most studies, patients have good long-term results, given the appropriate antibiotic regimen and, if indicated, closure of the leak. There were no cases of meningitis reported by either Wang et al. or Cammisa et al. (2,4).
Neurological Injury by Instrumentation and Implants
Neurological injury resulting from instrumentation and implants encompasses a variety of more specific causes. Injury can result anytime along the course of retracting the neural elements due to instruments used to prepare the disc space, injuries during implantation, and injuries afterward from migration of implants.
Nerve roots can be injured by retraction or stretch. Many techniques have been described for access to the disc space in posterior lumbar interbody fusion. The true central approach with laminectomy and without facetectomy has been shown to stretch the nerve roots and thecal sac in opposite directions, leading to stretch injuries and compression injuries against the pedicle wall. This has been termed “battered root syndrome.” Transforaminal approaches (TLIFs) have gained much more favor and lowered that risk. Rosenberg and Mummaneni (7) reviewed 22 TLIFs and found one transient L5 neuropraxia.
Root injury can also occur during the preparation for pedicle screws. Though rare, nerve root injury can occur if the inferomedial wall is breached by a pedicle finder. Jutte and Castelein (8) reviewed 105 consecutive instrumented fusions and attributed one case of transient L4 neuropraxia to an intraoperative medial wall breach with a pedicle finder.
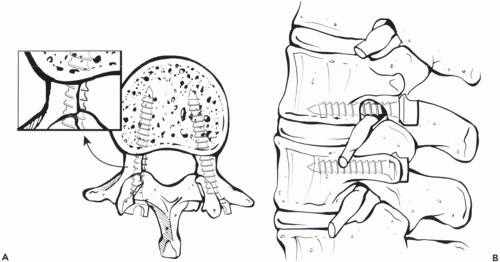
Pedicle screws themselves have been well-described as nerve root irritants when they breach the pedicle wall (Fig. 13.1). Lonstein et al. (9) reviewed 4,790 screws, and in patients with continued neurological deficits, found 11 screws (0.23%) in nine patients to be medial. After screw revision, six of the nine patients improved. In a study of 888 consecutive pedicle screws, Gundanna et al. (10) found that eight (0.90%) caused radiculopathy and/or deficits that were directly attributable to an inferomedial pedicle wall breach. All cases resolved (at least partially) upon revision of the implants. Interbody devices implanted from a posterior approach can migrate or dislodge posteriorly and impinge on the neural elements. Lin (11) reported this rate to be around 1%.
RELEVANT ANATOMY ASSOCIATED WITH SITE COMPLICATION
There are three layers of tissue over the neural elements in the lumbar spine: the dura mater, the arachnoid mater, and the pia mater. The dura mater is a relatively tough but thin layer of tissue that is the most superficial of the three layers. Laterally, the dura blends with the other layers to form the nerve root sleeve. Closely applied to the undersurface of the dura is a very thin and weak layer, the arachnoid mater. Between the dura and arachnoid, there is a potential space. Deep to the arachnoid is an actual space that contains the CSF. The pia is a thin layer that coats each nerve root individually as it floats in the CSF.
Though dural tears are often noted as the cause of CSF leaks, it requires a tear of both the dura and the arachnoid to have a leak. However, in the case of an isolated dural tear, the intact arachnoid is quite weak and cannot be relied upon to contain the pressure of the CSF without dural support, especially after Valsalva or coughing. Therefore, dural repair is strongly suggested.
Above the dura is the ligamentum flavum. This ligament is thick and tough, and in the case of degenerative disc collapse, this ligament can become redundant and even thicker. Typically, the ligamentum originates at the under-surface of the inferior part of the cephalad vertebral lamina and envelops the very superior aspect of the caudad vertebral lamina. There is a discontinuity of the ligament along the midline of the lamina, and there is no ligament under the cephalad portions of each vertebra; the dura is directly under the lamina. In the case of degenerative disc collapse, the ligamentum may infold and appear under more cephalad parts of the lamina, but it is not attached there.
Each neural foramen houses an exiting nerve root, except in the rare case of conjoined roots. Neural foramina are composed of the pedicles superiorly and inferiorly, and medially and posteriorly by the facet joints. Just outside the foramina are the dorsal root ganglia. Nerve roots separate out from the cauda equina and leave the midline, each in their respective nerve root sleeve, traversing the cephalad disc space. The roots hug their corresponding pedicles along the medial pedicle wall, just below the disc space, and exit just under their pedicle (12).
In the case of degenerative disease causing spinal stenosis, the thecal sac can be compressed centrally by the ligamentum dorsally, the central bulging disc ventrally, and the hypertrophic facets laterally. The traversing nerve root can be compressed by lateral recess stenosis or a posterolateral disc herniation. The exiting nerve root can be irritated by foraminal facet hypertrophy, or a lateral disc herniation.
HOW TO PREVENT COMPLICATIONS
Decompression
Antonacci and Eismont (13) outlined a stepwise surgical technique to minimizing neural and thecal injuries. First and foremost is to ensure adequate exposure. In the hunt for minimally invasive spinal surgery, maximal surgery is attempted in minimal exposure. Typically, spine surgery, regardless of approach, is a safe procedure, but injury in the periphery of the field is often difficult to repair, and injury can go undetected when trying to operate outside of the visual field. When injury is recognized, adequate lighting is mandatory to ensure an adequate repair, without entrapment of the neural elements.
Because instruments have become more complex, aggressive, sharp, and modular, it is important to not pass them over the wound. Only smooth and undamaged suction tips should be used around the dura. Bipolar electrocautery is essential to control epidural bleeding. Monopolar electrocautery should be avoided near the dura. Additionally, when being used away from the dura, other metallic instruments near the dura should be removed to avoid electrical arcing and inadvertent dural cautery.
Prior to insertion of sharp devices or the Kerrison rongeur, the space between the dura and the structure to be removed should be gently probed with a blunt instrument, and adhesions should be gently released. Bone removal should be done cautiously, with Kerrison rongeur footplates inserted parallel to the dura or nerve roots to avoid overly aggressive bites, preventing entrapment of neural elements, and to avoid leaving sharp points of bone that could pierce the dura, causing a postoperative leak.
When doing a decompressive laminectomy, the bone should be removed first at the inferior laminar edge using the ligamentum as protection deep. The Kerrison rongeur should be inserted with a motion tangential to the long axis of the dura, avoiding up- or downward pressure. Once the lamina is removed, the ligamentum can be removed. When removing the ligamentum or bone directly off of the dura, the rongeur should slide above the dura easily. If it does not, or the dura seems to move with the rongeur, there may be some adhesions and careful dissection with a Penfield no. 4 or other dissector of choice may be required. The ligamentum should not be pulled or torn off.
Performing foraminotomies can be tricky. The laminotomy should be adequately wide to allow access to the foramen and positive identification of the pedicles, but not so wide as to destabilize the pars and facets. Lateral recess stenosis can be difficult to remove without enough access. The nerve root should not be retracted; rather, it should be gently moved to the desired position. In cases of foraminal stenosis, the nerve root can be tethered in the foramen. Additionally, there is a possibility that the nerve roots are conjoined, which is probably more common than most surgeons suspect. To verify this would require a tracing of the aberrant nerve root proximally to its origin. It is important to recognize this situation to avoid resection of the conjoined root during lateral recess decompression.
To do the foraminotomy, the Kerrison rongeur should be inserted in the direction of the nerve root to reduce the chance of nerve transection. If possible, a Penfield No. 4
retractor or smooth suction tip should be used to retract the dura during the foraminotomy.
retractor or smooth suction tip should be used to retract the dura during the foraminotomy.
When using the high-speed burr, the tails of cotton pledgets should be noted, as they can easily shred the dura if caught and spun. The burr can be used to thin the bone in a level fashion, though more can be taken off the sides in the medial facet area where there is ligamentum below, so that the Kerrison bite is less bulky and more under control. If possible, the burr stroke should be from medial to lateral.
The thecal sac should not be manipulated above the L2 level. Additionally, the thecal sac should not be retracted more than 50% at any level below that. Bertrand has described battered root syndrome, in which postoperative radiculopathy occurs after laminectomy or laminotomy and is strongly suggestive of excessive root compression or retraction intraoperatively.
After decompression, the bony edges should be smooth. The anesthesiologist can be asked to do a Valsalva maneuver before closure. Hemostasis and bone bleeding should be minimized prior to closure.
Discectomy
Incising the intervertebral disc annulus should be done very carefully, steadily and, if possible, vertically. Magnification with loupes or a microscope and adequate lighting are necessary. Epidural bleeding around the disc can be brisk and may require bipolar electrocautery or pressure from thrombin-soaked gelfoam or cotton pledgets for control. Neural elements can be protected by a nerve root retractor placed medial to the annulotomy site prior to the annulotomy. The pituitary rongeur should be inserted into the disc space with the jaws closed and opened only when within the disc space. When scraping the disc space for loose fragments, the motion should be forward into the disc, not backward toward the dura.
Instrumentation for discectomies and end plate preparations for posterior and posterolateral interbody fusions have become increasingly aggressive. When using these instruments in the disc space, the dura must be protected at all times, and the traversing nerve root must be protected medially during a posterior interbody approach and laterally in a transforaminal approach. Additionally, the exiting nerve root must be protected in a transforaminal approach.
Interbody Instrumentation
The annulotomy must be clearly visualized and free of neural elements prior to the insertion of interbody devices. Many devices have serrated edges to enhance contact and stability with the end plates but also can catch and tear the dura or nerve root.
Posterior Instrumentation
Pedicle screws are intended to pass within the confines of the pedicle. Since the exiting nerve root travels along the medial, inferomedial, and inferior pedicular walls, these regions must not be breached. Based on preoperative radiographs, the diameter [anteroposterior (AP)] and approximate length (lateral) of pedicle screws can be assessed. Typically, a 6.5 mm × 45 mm screw is safe in a normal, adult lumbar spine.
Prior to instrumentation, the anatomic landmarks must be verified. Depending on the approach, the facet, transverse process, and lateral border of the pars can be seen or palpated. The sagittal angle of the pedicle screw should be determined by intraoperative lateral imaging after positioning the patient on the table. In the case of significant degenerative changes or atypical anatomy that obscures the screw entry point, laminotomies can be done to palpate the borders of the pedicle. The actual specific methods to localize are, of course, the surgeon’s preference (14).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree