In the past, renal failure was the leading cause of death after spinal cord injury (SCI). Today mortality from SCI has declined dramatically partly owing to the improved management of urologic dysfunction associated with SCI. The goals of bladder management in spinal cord injury patients are intended to (1) ensure social continence for reintegration into community, (2) allow low-pressure storage and efficient bladder emptying at low detrusor pressures, (3) avoid stretch injury from repeated overdistension, (4) prevent upper and lower urinary tracts complications from high intravesical pressures, and (5) prevent recurrent urinary tract infections. This article provides an overview of neurogenic bladder dysfunction associated with SCI and current management options.
The bladder has two main functions: the storage of urine under low intravesical pressure and periodic release of urine in a controlled coordinated manner during an acceptable time to void. The ability to maintain continence and release urine is under voluntary control mediated by neural input to the lower urinary tract (LUT) from centers located in the brain and spinal cord. A neurogenic bladder dysfunction is the result of disease or injury to the neural pathways or neuromuscular junctions controlling LUT functions, and commonly occurs after spinal cord injury (SCI).
In the past, renal failure was the leading cause of death after SCI . Today mortality from SCI has declined dramatically partly owing to the improved management of urologic dysfunction associated with SCI . The goals of bladder management in SCI patients are intended to (1) ensure social continence for reintegration into community, (2) allow low-pressure storage and efficient bladder emptying at low detrusor pressures, (3) avoid stretch injury from repeated overdistension, (4) prevent upper and lower urinary tracts complications from high intravesical pressures, and (5) prevent recurrent urinary tract infections. This article provides an overview of neurogenic bladder dysfunction associated with SCI and current management options.
Lower urinary tract anatomy and physiology
The LUT comprises the fundus, trigone, and neck of the bladder, the pelvic diaphragm, and the urethra. The bladder outlet consists of the bladder neck and urethral smooth and striated muscles. The urinary bladder is a four-layered musculomembranous structure composed primarily of smooth muscle cells that can contract when stretched. The four layers consist of (1) a three-layered detrusor muscle; (2) a serous layer; (3) a submucous, areolar layer; and (4) a thin mucous layer continuous with the ureteral and urethral linings. The structures primarily involved in the performance of bladder functions include the muscle of the fundus, bladder neck muscles, urethral smooth muscles, periurethral striated sphincter muscles, and striated pelvic muscles. The LUT urothelium acts as an active barrier, performing specialized sensory and signaling functions to regulate the chemical and physical environment.
Lower urinary tract innervation
Activity of the LUT must be coordinated during its storage and voiding phases. In the normally functioning LUT, the bladder outlet relaxes and bladder smooth muscle contracts during voiding while during the storage phase the detrusor relaxes and bladder neck contracts. Normal regulation of LUT functions is under voluntary control and involves a complex interplay between central and peripheral nervous system inputs ( Fig. 1 ).
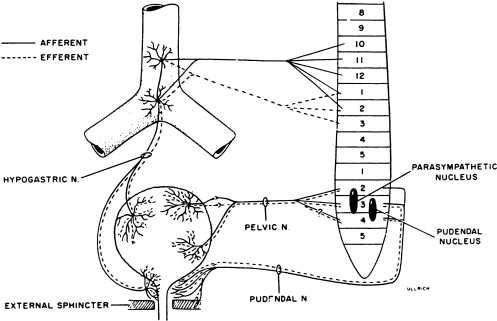
Peripheral innervation
Peripheral nervous system innervations include autonomic (sympathetic and parasympathetic) and somatic pathways ( Fig. 1 ). Parasympathetic innervation provides excitation to the smooth muscle of the bladder and inhibitory input to the urethral sphincter smooth muscle. Fibers originate from preganglionic cholinergic neurons in the intermediolateral region at the sacral levels, S2 through S4 of the spinal cord. Axons then travel by way of the pelvic nerves to ganglionic cells within the pelvic plexus and bladder wall. Excitatory transmission to the bladder wall is mediated mostly through the action of acetylcholine on the M3 muscarinic receptor subtype . The parasympathetic inhibitory input to the urethral sphincter, however, is mediated by the release of nitric oxide .
Sympathetic innervation provides inhibitory input to the bladder smooth muscle, excitation to the bladder neck, and modulation of parasympathetic ganglionic activity to the bladder. Thoracolumbar sympathetic pathways and prevertebral inferior mesenteric ganglia (T10–L2) travel primarily by way of the hypogastric nerves and also through the pelvic nerves. Noradrenaline release mediates inhibitory activity through β-adrenergic receptors on the bladder wall, whereas excitatory input to the bladder neck and urethra is through α1- and α2-adrenergic receptors .
Somatic efferent (motor) innervation from the Onuf’s nucleus in the anterolateral horn of the sacral spinal cord, S2 through S3, provides excitatory input to the striated muscles of the urethral sphincter. The efferent fibers travel by way of the pudendal nerves to reach the urethral sphincter, and excitatory activity is mediated by acetylcholine action on nicotinic receptors. LUT sensory (afferent) impulses that are conveyed to the central nervous system arise from receptors in the bladder and urethra. Impulses travel along the pelvic nerves and sacral spinal cord mainly by way of small myelinated (A-δ) and unmyelinated (C) fibers. The A-δ fibers have mostly mechanoreceptor functions responding to tension, whereas C-fibers have mostly chemoreceptor functions, responding to inflammatory or noxious stimuli within the LUT . Micturition is generally initiated through gradual bladder distension and contraction triggering the sensation of bladder filling and leading to A-δ afferent activation and subsequent active bladder contraction.
Central nervous system innervation
Voluntary control of voiding arises from the central nervous system, with centers located in both the brain and the spinal cord ( Fig. 2 ). Spinal cord pathways include efferent neurons, interneurons, and afferent neurons. Afferent fibers from the urinary bladder and urethra project to interneurons at the dorsal horn in the sacral spinal cord. Local connections involved in segmental spinal reflexes occur at that level. Other projections ascend through different pathways in the spinal cord to the pontine micturition center (PMC), periaqueductal gray matter, or ventral posterior nucleus of the thalamus and ultimately extend to the cerebral cortex.

Numerous other regions in the brain are believed to be involved in LUT control, including the medullary raphe nuclei, the locus coeruleus, the paraventricular nucleus of the hypothalamus, and neurons in the anterior hypothalamus . Efferent information from these suprapontine regions, including the frontal cortex and periaqueductal gray matter, then project to the PMC which functions as the site of integration of supraspinal input to regulate reflex micturition at the spinal level. Efferent pathways originating from the PMC then project to (1) motor neurons innervating the external urethral sphincter that originate from Onuf’s nucleus, (2) sacral parasympathetic preganglionic fibers, and (3) rostral lumbar sympathetic preganglionic fibers.
Lower urinary tract innervation
Activity of the LUT must be coordinated during its storage and voiding phases. In the normally functioning LUT, the bladder outlet relaxes and bladder smooth muscle contracts during voiding while during the storage phase the detrusor relaxes and bladder neck contracts. Normal regulation of LUT functions is under voluntary control and involves a complex interplay between central and peripheral nervous system inputs ( Fig. 1 ).
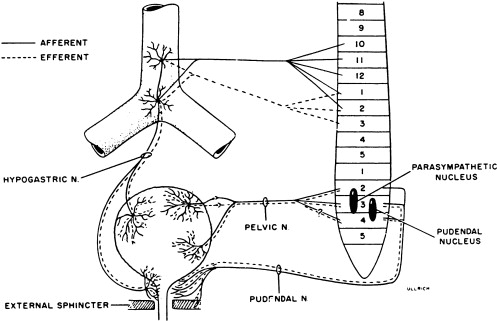
Peripheral innervation
Peripheral nervous system innervations include autonomic (sympathetic and parasympathetic) and somatic pathways ( Fig. 1 ). Parasympathetic innervation provides excitation to the smooth muscle of the bladder and inhibitory input to the urethral sphincter smooth muscle. Fibers originate from preganglionic cholinergic neurons in the intermediolateral region at the sacral levels, S2 through S4 of the spinal cord. Axons then travel by way of the pelvic nerves to ganglionic cells within the pelvic plexus and bladder wall. Excitatory transmission to the bladder wall is mediated mostly through the action of acetylcholine on the M3 muscarinic receptor subtype . The parasympathetic inhibitory input to the urethral sphincter, however, is mediated by the release of nitric oxide .
Sympathetic innervation provides inhibitory input to the bladder smooth muscle, excitation to the bladder neck, and modulation of parasympathetic ganglionic activity to the bladder. Thoracolumbar sympathetic pathways and prevertebral inferior mesenteric ganglia (T10–L2) travel primarily by way of the hypogastric nerves and also through the pelvic nerves. Noradrenaline release mediates inhibitory activity through β-adrenergic receptors on the bladder wall, whereas excitatory input to the bladder neck and urethra is through α1- and α2-adrenergic receptors .
Somatic efferent (motor) innervation from the Onuf’s nucleus in the anterolateral horn of the sacral spinal cord, S2 through S3, provides excitatory input to the striated muscles of the urethral sphincter. The efferent fibers travel by way of the pudendal nerves to reach the urethral sphincter, and excitatory activity is mediated by acetylcholine action on nicotinic receptors. LUT sensory (afferent) impulses that are conveyed to the central nervous system arise from receptors in the bladder and urethra. Impulses travel along the pelvic nerves and sacral spinal cord mainly by way of small myelinated (A-δ) and unmyelinated (C) fibers. The A-δ fibers have mostly mechanoreceptor functions responding to tension, whereas C-fibers have mostly chemoreceptor functions, responding to inflammatory or noxious stimuli within the LUT . Micturition is generally initiated through gradual bladder distension and contraction triggering the sensation of bladder filling and leading to A-δ afferent activation and subsequent active bladder contraction.
Central nervous system innervation
Voluntary control of voiding arises from the central nervous system, with centers located in both the brain and the spinal cord ( Fig. 2 ). Spinal cord pathways include efferent neurons, interneurons, and afferent neurons. Afferent fibers from the urinary bladder and urethra project to interneurons at the dorsal horn in the sacral spinal cord. Local connections involved in segmental spinal reflexes occur at that level. Other projections ascend through different pathways in the spinal cord to the pontine micturition center (PMC), periaqueductal gray matter, or ventral posterior nucleus of the thalamus and ultimately extend to the cerebral cortex.
Numerous other regions in the brain are believed to be involved in LUT control, including the medullary raphe nuclei, the locus coeruleus, the paraventricular nucleus of the hypothalamus, and neurons in the anterior hypothalamus . Efferent information from these suprapontine regions, including the frontal cortex and periaqueductal gray matter, then project to the PMC which functions as the site of integration of supraspinal input to regulate reflex micturition at the spinal level. Efferent pathways originating from the PMC then project to (1) motor neurons innervating the external urethral sphincter that originate from Onuf’s nucleus, (2) sacral parasympathetic preganglionic fibers, and (3) rostral lumbar sympathetic preganglionic fibers.
Normal micturition reflex
Normal bladder functions of storage and voiding are controlled by voluntary and reflex mechanisms. During the storage phase, the bladder must be able to expand slowly at low pressure until an appropriate bladder volume is reached, Sphincter activity must also be coordinated with bladder filling to allow adequate storage. Once threshold bladder volume is reached, the sacral reflex centers at S2 through S4 are stimulated with subsequent impulses sent from sacral spinal cord up to the PMC and frontal cortex. Efferent impulses sent from the brain stem stimulate bladder contraction, coordinated bladder neck relaxation, and closure of the ureteral valves, resulting in a sensation to void. If deemed socially appropriate, a conscious decision to void is initiated under the voluntary control of the frontal cortex which sends regulatory impulses to the external sphincter, by way of the corticospinal tract to the pudendal nerves. Voiding involves bladder wall contraction and relaxation of the internal and external sphincters. Conversely, if voiding is to be delayed, voluntary tightening of the external sphincter leads to associated bladder wall relaxation, internal sphincter contraction, and additional urine storage.
During the storage phase of bladder function, a sympathetic reflex activity through a local sacrolumbar spinal reflex pathway is triggered by afferent impulses in the pelvic nerves . This negative feedback mechanism contributes to the storage function of the bladder by increasing urethral outflow resistance, increasing bladder capacity, and decreasing the frequency and amplitude of bladder contractions. Once a threshold for bladder pressure is reached, a supraspinal inhibitory response, likely originating from the PMC, suppresses the vesicosympathetic reflex pathway and allows micturition to occur. As the bladder fills, afferent input from the bladder along with bulbospinal pathways from the pons maintain the normal, coordinated relationship between bladder and sphincter . This process further mediates activities of the striated muscles of the urethral sphincter and facilitates further bladder storage.
With voiding, afferent activity arising from tension receptors in the bladder activates neurons in the brainstem, the PMC, which functions as an “on/off” switch . Neuronal pathways between the rostral brain and pons then provide regulatory input through the brainstem, which then provides parasympathetic control of micturition through activation of sacral parasympathetic efferent pathways to the bladder and urethra.
Classification of neurogenic bladder in spinal cord injury
Many classification systems pertaining to neurogenic bladder have been devised. It has generally been classified based on neurologic, neurourologic, and functional classifications. The neurologic classification devised by Bors and Comarr can be applied to traumatic SCI. In this classification system, lesions are classified as either upper or lower motor neuron with respect to the anatomic location of the lesion relative to the sacral cord reflex centers.
Lower motor neuron
A lower motor neuron lesion is one that occurs at or below the conus medullaris ( Fig. 3 ). These lesions can affect efferent (motor), afferent (sensory), or both portions of the sacral arc pathway. Classic findings include an areflexic or hyporeflexic detrusor with a normal or underactive external sphincter. With a denervated or underactive external sphincter, coordination between detrusor contraction and sphincter relaxation occurs during bladder emptying (no detrusor–external sphincter dyssynergia [DESD]). If all the peripheral fibers below the level of injury are affected, loss of sacral reflexes occurs along with an areflexic bladder (a so-called “complete injury”). If, however, only some of the peripheral fibers are intact, a sacral reflex may be present with an areflexic bladder, suggesting an incomplete injury lower motor neuron lesion involving the conus, cauda equina, or peripheral nerve.
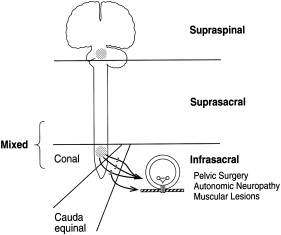
In a motor (efferent) neurogenic bladder, supraspinal regulatory transmission to afferent sacral nerves is spared, leading to preserved sensation of fullness, although normal sensation may be gradually lost because of recurrent overdistension injury. With strictly sensory (afferent) lesions, patients are able to void but have decreased sensation, which can lead to chronic overdistension and impaired emptying. Lesions involving motor and sensory pathways (the most typical) are associated with a mixture of symptoms. In a complete lesion at or below the conus medullaris, urodynamic studies will show an areflexic, low-pressure detrusor, absent EMG activity, elevated postvoid residual; and a competent bladder outlet.
Upper motor neuron
Upper motor neuron lesions can be of two types: (1) intracranial (suprapontine) lesions in which cortical input that inhibits detrusor contractility is interrupted while the PMC is intact, and (2) spinal (suprasacral or infrapontine) lesions pertaining to spinal cord injuries ( Fig. 3 ). Spinal lesions occur above the conus medullaris and spare the sacral reflex arc. The descending pontine (central) modulation of detrusor and sphincter activity is therefore disrupted, leading to DESD; detrusor–internal sphincter dyssynergia may also occur in lesions above T6.
Because sacral reflexes are present, independent sacral reflex activity leads to uninhibited bladder contraction during filling at a given volume threshold, and urinary incontinence with no sensation of bladder filling or urge to void. Additional findings include absent voluntary external sphincter control, spastic bladder, and often uncoordinated activity of bladder and external sphincter. Chronically elevated intravesical pressure from DESD, if untreated, often leads to upper urinary tract deterioration. Classic urodynamic findings include uninhibited bladder contraction, simultaneous contraction of detrusor and external sphincter, high intravesical pressure, and high postvoid residual.
Lower urinary tract conditions associated with neurogenic bladder
Several secondary conditions occur as a result of LUT dysfunctions in spinal cord injuries. These conditions are caused by impaired LUT regulation, and their feared complications, if not treated appropriately, include upper urinary tract deterioration and eventual renal failure.
Detrusor (bladder) overactivity
Detrusor overactivity often occur in suprasacral spinal lesions in which the sacral reflex arc is intact; the end result is an overactive, uninhibited bladder caused by disruption of central (pontine) modulation of detrusor and external sphincter. Uninhibited bladder contraction during filling leads to high intravesical pressures that can be aggravated by the presence of DESD. In severe cases that are unresponsive to other therapeutic interventions, surgical defunctionalization of the detrusor can be considered, and can be in the form of either augmentation enterocystoplasty, bladder autoaugmentation, or conduit/continent diversions.
Bladder wall compliance
Another associated bladder dysfunction seen in neurogenic conditions is either an increase or decrease in bladder wall compliance; this is the ratio of a change in bladder volume to the associated change in intravesical pressure and is usually obtained from urodynamic study. A poorly compliant bladder distends with high intravesical pressure at relatively low volumes, and may lead to vesicoureteral reflux and places the upper urinary tract at even greater risk for deterioration . A highly compliant bladder, conversely, is seen associated with a hyporeflexic or areflexic bladder as seen in lower motor neuron injuries. Intravesical pressures are generally low and, therefore, not harmful. Patients are usually unable to void and strict clean intermittent catheterization (CIC) is required to avoid overdistension injuries.
High leak-point detrusor pressure
The detrusor leak-point pressure (DLPP) is the maximum detrusor storage pressure at which leakage occurs from the bladder during passive filling and is determined from urodynamic studies. Sustained high detrusor pressure often results from a poorly compliant bladder and, when left uncorrected, further places the upper urinary tract at risk . DLPP exceeding 40 cm H 2 O is believed to place the upper tract at especially higher risk for deterioration .
Vesicoureteral reflux
Vesicoureteral reflux results from high DLPP and high intravesical pressure from low bladder compliance with or without detrusor-sphincter dyssynergia, and is associated with a higher risk for urinary tract infection and upper tract deterioration . Refluxing into the ureters and up to the kidneys leads to renal damage from pyelonephritis or ischemic injuries, with eventual renal scarring.
Detrusor-external sphincter dyssynergia
DESD is an intermittent or continuous involuntary contraction of the urethral sphincter during detrusor contraction. It is a common occurrence in suprasacral spinal cord lesions . DESD has well-documented clinical significance, such as high detrusor pressure, vesicoureteral reflux, and upper tract deterioration . Significant bladder outlet obstruction with associated high detrusor pressure may require transurethral sphincterotomy to reduce the detrusor pressure during voiding. Alternative treatment options, such as botulinum-A toxin injection into the external urethral sphincter, have also been used .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





