Abstract
Background
Botulinum toxin type A manages spasticity disorders in neurological central diseases. Some studies have reported that it might induce muscle changes.
Methods
We present a literature review abiding by the PRISMA statement guidelines. The purpose was to explore the structural and passive biomechanical muscle properties after botulinum toxin type A injections in healthy and spastic limb muscles, on animals and humans, as well as methods for evaluating these properties. We searched the PubMed and Cochrane Library databases using the following keywords: “Botulinum toxin” AND (“muscle structure” OR “muscle atrophy”) and, “Botulinum toxin” AND “muscle elasticity”.
Results
From the 228 initially identified articles, 21 articles were included. Histological analyses were performed, especially on animals. A neurogenic atrophy systematically occurred. In humans, one year after a single injection, the histological recovery remained incomplete. Furthermore, 2D ultrasound analyses showed a reduction of the gastrocnemius thickness and pennation angle. MRI volumetric analysis evidenced muscular atrophy six months or one year after a single injection. Passive muscle stiffness depends on these structural changes. On the short term, the biomechanical analysis showed an elastic modulus increase in animals whereas no change was recorded in humans. On the short term, ultrasound elastography imaging showed a decreased elastic modulus.
Discussion
To date, few data are available, but all show a structural and mechanical muscle impact post injections, specifically muscle atrophy which can linger over time. Further studies are necessary to validate this element, and the possibility of change must be taken into account particularly with repeated injections. Thus, in clinical practice, 2D ultrasound and ultrasound elastography are two non-invasive techniques that will help physicians to develop an efficient long term monitoring.
1
Introduction
The spastic paretic muscle is subject to structural changes compared to healthy skeletal muscles. These changes are independent from the initial etiology of the neurological lesion. The literature describes an increased variability of the size and type of muscle fibers, decreased numbers of sarcomeres, proliferation of the extracellular matrix (ECM) with an increased collagen concentration . When the first motor neuron is affected, muscle retraction can get settled under the influence of two main factors. The disuse of the paretic muscle or “functional immobilization”, which initiates muscle atrophy and reduces the number of sarcomeres by a disequilibrium of the protein-proteolysis synthesis balance in favor of the proteolysis . Chronic muscle hyperactivity maintains the muscle in a short position, reducing the longitudinal tension . Thus, the shortening of the muscle fibers and accumulation of conjunctive tissue are responsible of these changes in biomechanical viscoelastic properties of the muscles, with a decreased passive extension capacity .
Muscle changes contribute to functional impairments. Gait speed and step length have been significantly correlated to passive mechanical properties of the plantar flexor muscles, determined by the measure of the passive torque/joint angle ratio of the talocrural joint . For Dietz and Sinkjaer, the spastic muscle at rest is submitted to an overexcitability of the alpha motor neuron (presynaptic inhibition and increased activity of type Ia ascending fibers) with little changes during voluntary contractions. Thus, hyperreflexia is hardly involved in pathologic spastic movements .
Injections of botulinum toxin type A (BoNtA) are a first-line therapeutic method to treat focal spasticity ). Their action is triggered by the fixation on the SNARE proteins and inhibited release of acetylcholine (ACh) from the presynaptic terminals. The therapeutic objective is to decrease reflex muscle overactivity and fight muscle hypertonia. The functional benefit will affect gait patterns and movement amplitude . However, the impact of BoNtA injections on muscle structure and the stretching capacity of muscle tissue have rarely been reported in the literature. The challenge is to differentiate the consequences related to spastic paresis from those linked to the injections of botulinum toxin. We propose a review of the literature with the following objectives:
- •
analyze changes in the structure and stiffness of muscle tissue described after an injection of botulinum toxin in one muscle of the limbs;
- •
discuss the evaluation methods used.
2
Methodology
A systematic review of the literature was conducted abiding by the PRISMA recommendations ( www.prisma-statement.org ). We searched the Pubmed and Cochrane Library databases, using the following keywords
“Botulinum toxin” AND (“muscle structure” OR “muscle atrophy”) and, “Botulinum toxin” AND “muscle elasticity”. Articles stemming from this research were independently put aside by 2 authors (LM and BP) and were then evaluated. Articles were kept if they met the following criteria:
- •
the study focused on the analysis of a striated skeletal muscle of the limbs, paretic spastic or healthy (to differentiate changes related to spastic paresis), in men and humans;
- •
the study analyzed the consequences of injections of botulinum toxin on muscle structure and/or muscle tissue stiffness;
- •
evaluation methods in the fields of histological, mechanical and medical imaging analyses were described;
- •
the full manuscripts were published in English between 1990 and October 2014. References of the articles included were used to eventually complete the selection. In case of disagreement, a decision was taken after further discussion.
The methodological quality of the articles was evaluated using a specific scale developed based on the STROBE (Strengthening the Reporting of Observational studies in Epidemiology) principles . Each item was categorized, and the maximum global score is 28 ( Table 1 ).
| Q1 | Is there in the abstract an explanation of what was done and found? |
| Q2 | Is the scientific context clearly explained? |
| Q3 | Are the objectives clearly stated? |
| Q4 | Is the sampling size indicated? |
| Q5 | If yes, is the sampling size statistically justified? |
| Q6 | Are the characteristics of the subjects (height, weight, sex, healthy or pathologic subject) described? |
| Q7 | What is the design of the study? (0: retrospective study; 1: case study; 2: prospective study). |
| Q8 | Is there a control group? (0: no, 1: contralateral member or non-randomized control group, 2: randomized control group). |
| Q9 | How long is the follow up? (0: ≤ 1month; 1 ≤ 6 month; 2 ≥ 1 year) |
| Q10 | Is the reliability of the evaluation method clearly described? |
| Q11 | Are the results interpretable? |
| Q12 | Are the limitations of the study discussed? |
| Q13 | Is the conclusion clearly stated? |
2
Methodology
A systematic review of the literature was conducted abiding by the PRISMA recommendations ( www.prisma-statement.org ). We searched the Pubmed and Cochrane Library databases, using the following keywords
“Botulinum toxin” AND (“muscle structure” OR “muscle atrophy”) and, “Botulinum toxin” AND “muscle elasticity”. Articles stemming from this research were independently put aside by 2 authors (LM and BP) and were then evaluated. Articles were kept if they met the following criteria:
- •
the study focused on the analysis of a striated skeletal muscle of the limbs, paretic spastic or healthy (to differentiate changes related to spastic paresis), in men and humans;
- •
the study analyzed the consequences of injections of botulinum toxin on muscle structure and/or muscle tissue stiffness;
- •
evaluation methods in the fields of histological, mechanical and medical imaging analyses were described;
- •
the full manuscripts were published in English between 1990 and October 2014. References of the articles included were used to eventually complete the selection. In case of disagreement, a decision was taken after further discussion.
The methodological quality of the articles was evaluated using a specific scale developed based on the STROBE (Strengthening the Reporting of Observational studies in Epidemiology) principles . Each item was categorized, and the maximum global score is 28 ( Table 1 ).
| Q1 | Is there in the abstract an explanation of what was done and found? |
| Q2 | Is the scientific context clearly explained? |
| Q3 | Are the objectives clearly stated? |
| Q4 | Is the sampling size indicated? |
| Q5 | If yes, is the sampling size statistically justified? |
| Q6 | Are the characteristics of the subjects (height, weight, sex, healthy or pathologic subject) described? |
| Q7 | What is the design of the study? (0: retrospective study; 1: case study; 2: prospective study). |
| Q8 | Is there a control group? (0: no, 1: contralateral member or non-randomized control group, 2: randomized control group). |
| Q9 | How long is the follow up? (0: ≤ 1month; 1 ≤ 6 month; 2 ≥ 1 year) |
| Q10 | Is the reliability of the evaluation method clearly described? |
| Q11 | Are the results interpretable? |
| Q12 | Are the limitations of the study discussed? |
| Q13 | Is the conclusion clearly stated? |
3
Results
3.1
Selection of the studies
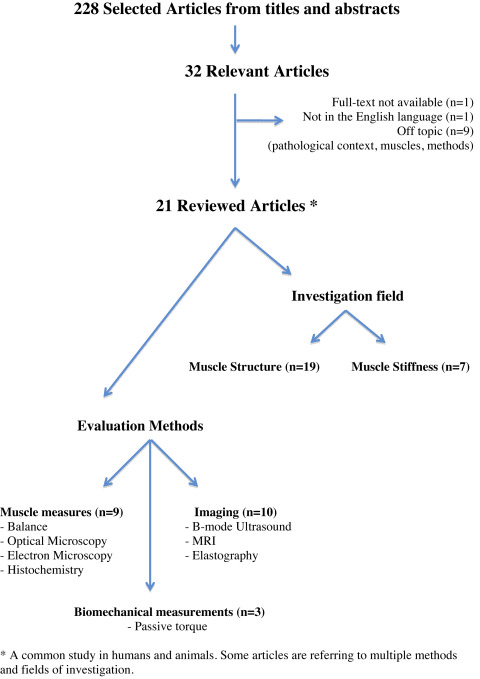
In all, 228 articles were initially identified ( Fig. 1 ). Thirty-two articles were deemed relevant after reading the titles and abstracts. Twenty-one were included in the review (11 were excluded because they did not meet the selection criteria). Fourteen studies focused on the structural analysis, 3 studies on muscle stiffness analysis and 4 studies on both structure and stiffness analysis. Overall, there were very few studies, with often a restricted population sample (from 1 to 56 patients in men) and various exploration methods.
3.2
Quality of the reviewed articles
The quality of the reviewed articles is summed up in Table 2 . It is highly variable. Most studies were prospective ones, except for 2 case studies and a retrospective study . The descriptive quality of the experimental protocol, results as well as their interpretation and conclusion was adequate in most studies. The reproducibility of evaluation method was rarely described. No study proposed sample size calculations. The follow-up duration was quite short in most studies (≤ 3 months).
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Total (max = 28) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alhusaini et al. (2011) | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 0 | 1 | 1 | 2 | 2 | 2 | 21 |
| Boyaci et al. (2014) | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 1 | 0 | 0 | 0 | 2 | 1 | 15 |
| Choi et al. (2007) | 2 | 1 | 1 | 2 | 0 | 2 | 2 | 1 | 0 | 1 | 1 | 0 | 2 | 15 |
| Dodd et al. (2005) | 2 | 2 | 2 | 0 | 0 | 1 | 2 | 2 | 1 | 1 | 1 | 0 | 2 | 16 |
| Fortuna et al. (2011) | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 20 |
| Frick et al. (2007) | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 2 | 1 | 1 | 2 | 1 | 2 | 20 |
| Haubruck et al. (2012) | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 0 | 1 | 2 | 2 | 2 | 21 |
| Kwon et al. (2012) | 1 | 1 | 1 | 2 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 11 |
| Legerlotz et al. (2009) | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 0 | 1 | 2 | 0 | 2 | 19 |
| Ma et al. (2004) | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 23 |
| Park and Kwon (2012) | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 0 | 0 | 1 | 1 | 2 | 2 | 17 |
| Picelli et al. (2012) | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 2 | 2 | 18 |
| Schroeder et al. (2009) | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 1 | 2 | 1 | 2 | 0 | 2 | 19 |
| Shaikh et al. (2014) | 1 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 1 | 1 | 1 | 1 | 2 | 17 |
| Stone et al. (2011) | 2 | 2 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 1 | 1 | 1 | 2 | 16 |
| Thacker et al. (2012) | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 1 | 0 | 1 | 2 | 1 | 2 | 18 |
| Tok et al. (2011) | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 0 | 1 | 0 | 1 | 1 | 2 | 16 |
| Tsai et al. (2010) | 2 | 2 | 1 | 2 | 0 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 2 | 18 |
| Van Campenhout et al. (2013) | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | 2 | 2 | 1 | 19 |
| Vasilescu et al. (2010) | 1 | 2 | 1 | 2 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 11 |
| William et al. (2013) | 2 | 2 | 1 | 2 | 0 | 2 | 0 | 0 | 1 | 2 | 2 | 2 | 2 | 18 |
3.3
Literature analysis ( Table 3 )
The methodological variability among the small number of studies, made it mandatory to conduct an evaluation based on changes in muscle structure and passive mechanical properties and according to the exploration techniques used .
| Author Year | Human/Animal | Control group | Age | Population (number) | Neurological Condition | Muscle system | BoNtA Number of injections and dose | Measurement tool | Study criteria | Changes | First–Last evaluation | First changes | Recovery |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alhusaini et al., 2011 | Human | No | 4 to 10 years | 16 | CP | TS + TA + TCJ unit | Botox, 1, ? | Dynamometer–Potentiometer | TS + TA + TCJ unit stiffness | None | 6 weeks–6 weeks | None | ? |
| Boyaci et al., 2014 | Human | Yes | Avg: 49 ± 16 months | 16 | CP | GM, GL, So | Botox, 1, 1,5U/kg in GM | B-mode US | Muscle thickness | None in injected GM Increase in GL, Soleus | 1week pre-BoNtA–4 weeks post-BoNtA | 4 weeks | ? |
| Sonoelastography | Strain ratio | Decrease in injected GM and in GL | |||||||||||
| Red Pixel intensity | Decrease in injected GM and in GL | ||||||||||||
| Choi et al., 2007 | Rat | Yes | Mature, 4 year | 80 | Healthy | RF | ? 4 groups 0, 1, 3, 9 ng/kg/day, daily for 4 weeks | Balance | Body weight | Dose-dependent weight loss | D1–4 weeks | Dose-dependent | No |
| Histology | Muscle structure | Decrease of muscle fiber diameter Increase of nuclei number Increase of collagen Increase of adipocytes | |||||||||||
| Dodd et al., 2005 | Rat | Yes + contralateral limb | Mature, 4 months | ? | Healthy | TS | Dysport, 1 4 groups: 3 U, 6 U, 12 U, 18 U | Balance | Muscle mass | Decrease | D1–D67 | D67 | No |
| Histochemistry | MHC isoforms | Decrease MCH IIx → IIa et I | |||||||||||
| Fortuna et al., 2011 | Rat | Yes | Mature, 1 year | 20 | Healthy | RF, VL, VM | Botox, monthly, 3,5 U/kg | Balance | Muscle mass | Decrease | 1 months–6 months | 1 month | ? |
| Histology | Contractile proteins % | Decrease | 3 months | ||||||||||
| Frick et al., 2007 | Rat | Yes + contralateral limb | Mature | 30 | Healthy | TA | Botox, 1 3 groups: 0,625 U, 2,5 U, 10 U | Balance | Muscle mass | Dose-dependent decrease | 128 days–128 days | 128 days | ? |
| Haubruck et al., 2012 | Rat | Yes | Mature | 36 | Healthy | GCM + TA + calcaneus unit | ?, 1, 6 U/kg | Dynamometer + lenght measure | GCM + TA + calcaneus unit stiffness | Decrease | 8 days–8 days | 8 days | ? |
| Kwon et al., 2012 | Human | Yes | 28 years | 1 | CP | GCM, So | Botox, 1, 20 U | B-mode US | Muscle thickness | Increase | 4 weeks–4 weeks | 4 weeks | ? |
| Sonoelastography | Elastic modulus with color maps | Softer muscles | |||||||||||
| Legerlotz et al., 2009 | Rat | Yes | Juvenile, 4 weeks | 30 | Healthy | GCM | Botox, 1, 13 U/kg | Balance | Muscle mass | Decrease | 3 weeks–3 weeks | 3 weeks | ? |
| Histochemistry | MHC isoforms | MHCIIb → MHCIIx or IIa | |||||||||||
| Gel Electrophoresis | Titin content | Decrease | |||||||||||
| Ma et al., 2004 | Rat | Yes | Juvenile, 1 month | 34 | Healthy | GM, GL | ?, 1 6 U/kg | Balance | Muscle mass | Decrease | 1 week–1 year | 1 week | 6 months |
| Histology: ME | NMJ morphometry: width | Increase | 2 months | 1 year | |||||||||
| Park et Kwon, 2012 | Human | No | Avg: 57 ± 22 months | 17 | CP | GM, GL | Botox, 1, 20 U/muscle | Sonoelastography | RTS score | Decrease | 4 weeks | 4 weeks | ? |
| Red pixel intensity | Decrease | ||||||||||||
| Picelli et al., 2012 | Human | No | Avg: 59 ± 14 ans | 56 | Stroke | GM, GL | ?, 1, 250 U/GCM | B-mode US | Muscle echogenicity, Heckmatt Scale | No echogenicity changes Injections less effective depending on echogenicity | 4 weeks–4 weeks | 4 weeks | ? |
| Schroeder et al., 2009 | Human | Contralateral limb | 31 and 47 years | 2 | Healthy | GL | Xeomin, 1, 75 U | MRI | Muscle signal | High signal intensity in STIR sequence | 3 months–12 months | 3 months | No |
| Muscle cross-sectional area | Decrease | ||||||||||||
| OM | Muscle structure | Neurogenic atrophy Reinnervation | |||||||||||
| EM | Muscle ultrastucture | ||||||||||||
| Shaikh et al., 2014 | Human | Yes | Avg: 47 years (min: 26–max: 63) | 12 | Piriformis syndrome | Piriformis | Botox, inconstant injections number, 100 U | MRI | Maximal muscle thickness | Decrease | Inconstant Avg: 7,3 ± 5,2 months post-injection | ? | No |
| Muscle volume | Decrease | ||||||||||||
| Fatty infiltration | Increase | ||||||||||||
| Stone et al., 2011 | Mouse | No | ? | 140 | Healthy | GM, GL | Botox, 1, inconstant doses and volumes | Balance | Muscle mass | Dose- and volume-dependant decrease | 4 weeks–12 weeks | 4 weeks | 12 weeks |
| Thacker et al., 2012 | Rat | Contralateral limb | Mature | 24 | Healthy | TA | Botox, 1, 6 U/kg | Dynamometer + Length measure (muscle fibers) | Elastic modulus | Increase | 1 month–1 month | 1 month | ? |
| Histology | Collagen content | Increase | |||||||||||
| Tok et al., 2011 | Human | Contralateral limb | Avg: 55 ± 14 years | 26 | Stroke | GM, GL | ?, 1, ? | B-mode US | Pennation angle | Decrease | 10 days–2 months | 2 months | ? |
| Fascicular lenght | Increase | ||||||||||||
| Muscle thickness | Decrease | ||||||||||||
| Muscle compressibility | None | ||||||||||||
| Tsai et al., 2010 | Human | No | ? | 5 | Calf asymetry | TS | Botox, 1, 20 ng calf | Meter | Maximal calf circumference | Decrease | 4 weeks–26 weeks | 4 weeks | 26 weeks |
| Rat | Yes | ? | 5 | Healthy | GCM | 3 groups: 1,5 ng/kg 6 months repeated 1,5 ng/kg 6 months repeated 1 ng/kg | EM | Muscle ultrastucture | Sarcomere distorsion | 1 week–52 weeks | 1 week | 26 weeks | |
| Balance | Muscle mass | Decrease | |||||||||||
| Van Campenhout et al., 2012 | Human | No | Avg: 12 years | 7 | CP | Proximal psoas Distal psoas | Botox, 1, 2 U/kg/psoas | MRI | Muscle volume | Decerase (proximal injected psoas) None (distal injected psoas) | 2 months–6 months | 2 months | No |
| Vasilescu et al., 2010 | Human | Contralateral limb | 3–10 years | 7 | CP | Inconstant | ? | B-mode US | Muscle echogenicity Aponevrosis echogenicity, Diamètre musculaire | No description | ? | ? | ? |
| Sonoelastography | Elasticity pattern with color map | Softer muscles | |||||||||||
| William et al., 2013 | Human | No | 5–11 years | 15 | CP | GCM, So | Botox, 1, inconstant doses | MRI | Muscle volume | Decrease in injected muscles Increase in soleus muscle | 5 weeks | 5 weeks | ? |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




