Management of Infected Shoulder Arthroplasty
Eric T. Ricchetti
The author certifies that he has no commercial associations (e.g., consultancies, stock ownership, equity interest, and patent/licensing arrangements) that might pose a conflict of interest in connection with the submitted chapter.
INTRODUCTION
Infection after shoulder arthroplasty is a devastating complication with significant patient morbidity. The incidence of infection following shoulder arthroplasty has been reported between 0% and 4% in the literature, with rates up to 15% for revision arthroplasty.4,10,17,30,49 Recently, Bohsali et al performed a systematic review of all studies documenting complications following unconstrained total shoulder arthroplasty (TSA).4 Infection represented 4.6% of all complications, with an overall prevalence of 0.7%. Another large, recent systematic review reported an overall incidence of infection following unconstrained TSA of 1.1%, representing 2.9% of all complications in the review.17 Unlike the extensive data in the hip and knee literature that exists on management of infected arthroplasty, much less has been reported on this problem in the shoulder, with varying treatment protocols and outcome measures. This lack of evidence can make successful management challenging, and the treatment algorithms are still evolving. To further complicate matters, more recent literature has suggested that less virulent organisms, particularly Propionibacterium acnes, may be a significant cause of infections after shoulder arthroplasty and may be less clinically evident and more difficult to diagnose.36,37,42,49,58 The optimal treatment strategy for these less aggressive infections is not yet known.
The purpose of this chapter is to review the management of infection following shoulder arthroplasty, including patient evaluation, diagnostic studies, treatment strategies, and outcomes.
PATIENT EVALUATION
History and Physical Examination
An accurate history of the patient’s postoperative course following shoulder arthroplasty is critical in determining the presence of an infection, as well as its onset. Infections may be classified as acute (less than 3 months after surgery), subacute (between 3 and 12 months after surgery), and late/chronic (greater than 1 year after surgery), based on the timing of presentation following surgery.4,49,51 A classification system based on the time of onset of infection following arthroplasty has also been reported in the hip and knee literature, with four types described; type 1 is the presence of positive cultures at the time of revision arthroplasty, type 2 is an acute infection detected within 30 days of arthroplasty, type 3 is an acute hematogenous infection that may occur at any time, and type 4 is a chronic infection.44 This classification system can help guide management options, as discussed below.
Pain is the most common presenting complaint in the setting of an infected shoulder arthroplasty, and its onset may assist in determining when the infection began. In the typical scenario of an infection developing at the time of primary arthroplasty, patients will often describe a history of difficulties from the onset, with a sense that their shoulder never got better following shoulder arthroplasty or never had even a short period of improvement. Unlike other aseptic conditions, such as component loosening or instability, patients with infection typically report pain that is constant, rather than related to a particular activity. Stiffness is also commonly reported in combination with pain.10,49 Patients may note difficulty in gaining range of motion in their shoulder during the postoperative period, and this stiffness may further intensify the pain symptoms. Overt clinical signs of infection, such as fevers, sweats, and chills, or purulent drainage from the incision site, may not be present as infections can be either low grade or subclinical. However, it is important to inquire about any history of wound drainage during the initial postoperative period as another clue that may raise suspicion for infection. Hematoma formation after shoulder arthroplasty, particularly if necessitating an irrigation and debridement procedure, has been associated with the development of positive cultures and subsequent deep infection.6 The patient’s medical history should also be thoroughly detailed to determine risk factors for infection that may have been present preoperatively. Risk of postoperative infection is often increased due to either host-related factors (diabetes mellitus, rheumatoid arthritis, systemic lupus erythematosus, prior surgery, remote source of infection, malnutrition, obesity, age, etc.) or extrinsic factors (chemotherapy, radiation therapy, systemic corticosteroids, repeated steroid injections, etc.) that predispose to immunosuppression.4,10,32,48,49,57
The physical examination of the shoulder should start with the inspection of the patient’s prior incision(s). Overtly concerning signs include redness or cellulitis, swelling, purulent drainage, or a chronic sinus tract; however, the incision(s) may look benign in a low-grade or subclinical infection. Signs of muscle atrophy, particularly in the deltoid and rotator cuff muscles, should also be noted as possible evidence of another problem, such as a rotator cuff tear or nerve injury. Tenderness can be noted when palpating about the shoulder, particularly along the glenohumeral joint line. Range of motion of the shoulder will demonstrate signs of stiffness, which is typically present in all planes. End range pain is usually associated with loss of motion. Discrepancies in passive and active range of motion should also be determined, and can raise concern for an associated rotator cuff or nerve injury. Strength testing of the shoulder will also bring out evidence of a possible rotator cuff problem or nerve injury.
DIAGNOSTIC STUDIES
As with prosthetic infections in other joints, further diagnostic work-up can include measurement of serum C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and white blood cell (WBC) count; radiographic studies to evaluate for humeral or glenoid component loosening; advanced imaging studies such as indium In-111-labeled WBC scan; and joint aspiration.4,57 Multiple abnormal findings on preoperative studies raise suspicion for a prosthetic infection, but no single test has been shown to reliably diagnose prosthetic infection on its own.14 CRP and ESR are frequently elevated in cases of infected shoulder arthroplasty,10,11,41,49 but are nonspecific markers of inflammation. In the early postoperative period, CRP and ESR may normally be elevated and, therefore, less useful. Little information exists on when these blood tests normalize specifically following shoulder arthroplasty, however, in the hip and knee literature, it has been reported that CRP typically peaks on the second postoperative day, and normalizes within 2 weeks of an uncomplicated surgery.35,42,47,56 ESR declines more slowly, and one or both may remain elevated for longer periods in patient with inflammatory arthropathies, such as rheumatoid arthritis.14,31,42,47 Recently, other serum markers have been evaluated for their use as preoperative tests in the diagnosis of prosthetic infection.14 Interleukin-6 (IL-6), in particular, has shown promise as a more accurate test than CRP, ESR, and WBC count,2 with early data on its use in shoulder arthroplasty also consistent with this finding.9
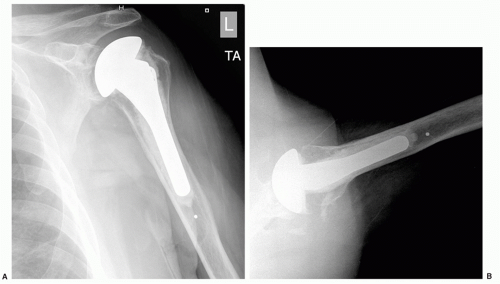
Radiographs of the shoulder can be very useful in the diagnosis of infection in the nonacute setting. The presence of radiolucencies around one or both components or gross component loosening should raise concerns, particularly if the history does not suggest an aseptic cause or if lucencies develop over a relatively short period of time (Fig. 25-1). Periosteal new bone formation may be another radiographic sign suggestive of infection.47 Ultrasonography and magnetic resonance imaging (MRI) may be useful in identifying a fluid collection near an implant, but artifact from the prosthesis may distort the images.42 A technetium Tc-99 bone scan or indium In-111-labeled WBC scan may be useful imaging studies in the diagnosis of infection, particularly if other diagnostic tests are equivocal.14 However, their accuracy has been mixed in reports on use in shoulder arthroplasty and such tests may give false-positive results in patients with inflammatory arthropathy.10,11,51 More recently, positron emission tomography (PET) has shown promise as an adjunctive imaging tool in the diagnosis of periprosthetic infections.7,42
Joint aspiration is an important part of the evaluation process for infected shoulder arthroplasty. Aspiration may be performed blindly or fluoroscopically guided, in order to increase the odds that an accurate fluid sample is obtained from the glenohumeral joint. The aspiration can be performed from an anterior or posterior approach and should be done outside of any areas of cellulitis. It is critical when an aspiration is obtained that the patient is not currently on any antibiotics that may cause a false-negative result, and that the aspirate is cultured for an appropriate length of time. Patients should be off of antibiotics for a minimum of 2 to 3 weeks to obtain an accurate culture.14 The most commonly isolated organisms from an infected shoulder prosthesis are Staphylococcus aureus, coagulasenegative Staphylococcus or Staphylococcus epidermidis, and P. acnes.10,17,26,49,51 Routine culture times may not be held long enough to detect less virulent infections, particularly P. acnes, that are a common cause of infection following shoulder arthroplasty.10,26,49,51 P. acnes, previously thought to be a culture contaminant, has been found to have a predilection for the shoulder.36,37,42,49,58 Cultures at our institution
are held for 14 days to increase the likelihood of detecting these more indolent infections, but incubation times of up to 21 days have been reported as necessary for P. acnes.36,37,42 Synovial fluid WBC count with WBC differential from the aspirate can also be useful in the diagnosis of infection, however, no literature with regards to cutoff levels for infection in shoulder arthroplasty is currently available. In the knee arthroplasty literature, it has been shown that combining synovial fluid WBC count and differential, either with each other or with ESR and CRP, can improve their diagnostic value in the setting of periprosthetic infection, and cutoff values as low as >1,100 cells/10−3 cm3 for WBC count and >64% for neutrophil percentage on differential can be used to diagnose prosthetic infection.16 Inflammatory proteins, such as CRP and IL-6, are also now being measured in synovial fluid as potential diagnostic tests for prosthetic infection.27
are held for 14 days to increase the likelihood of detecting these more indolent infections, but incubation times of up to 21 days have been reported as necessary for P. acnes.36,37,42 Synovial fluid WBC count with WBC differential from the aspirate can also be useful in the diagnosis of infection, however, no literature with regards to cutoff levels for infection in shoulder arthroplasty is currently available. In the knee arthroplasty literature, it has been shown that combining synovial fluid WBC count and differential, either with each other or with ESR and CRP, can improve their diagnostic value in the setting of periprosthetic infection, and cutoff values as low as >1,100 cells/10−3 cm3 for WBC count and >64% for neutrophil percentage on differential can be used to diagnose prosthetic infection.16 Inflammatory proteins, such as CRP and IL-6, are also now being measured in synovial fluid as potential diagnostic tests for prosthetic infection.27
If a patient is taken to the operating room for revision surgery, intraoperative tissue samples should be obtained for Gram stain, culture, and pathology, including frozen section. This is particularly important when a preoperative aspiration is negative, but clinical suspicion for infection remains high. Patients should again be off of antibiotics for a minimum of 2 to 3 weeks to obtain accurate intraoperative cultures. Appropriate cultures should be sent and incubated for an adequate length of time, including aerobic and anaerobic cultures (incubate up to 21 days), fungal (4 weeks), and mycobacterium (8 weeks).42 Intraoperative Gram stain and culture still have been reported to be negative in otherwise clinically confirmed cases of infected shoulder arthroplasty.10,29,49,51 This may be due to insufficient tissue samples, inadequate culture length, and remaining on or failing to discontinue antibiotics early enough before surgery. Tissue samples should ideally be obtained from the joint capsule, the prosthesis-bone interface, and from the medullary canal to have adequate material for both culture and histology. Intraoperative frozen section can provide further evidence of infection, with a criterion of more than five polymorphonuclear leukocytes per high-powered field typically considered positive for prosthetic infection.11,30,34,42,51,53 Specific cutoffs for shoulder arthroplasty have not been reported, however, and threshold criteria may need to be lowered for less virulent organisms. Implant sonication is a new diagnostic technique in which bacteria can be detected on the removed prosthesis by identifying them on the adherent biofilm. Implant sonication and polymerase chain reaction (PCR) techniques have been shown to be more sensitive than intraoperative tissue samples in bacterial identification, but the techniques have not yet gained widespread use.3,37,42
The diagnosis of infection following shoulder arthroplasty can be challenging in certain cases because overt clinical signs and symptoms may not be present, radiographic imaging may be normal, and blood work such as CRP, ESR, and WBC, or joint aspirates can be negative.15,30,53 Topolski et al. reported on the results of preoperative studies and intraoperative histology among patients without overt signs of infection, who underwent revision shoulder
arthroplasty and had positive intraoperative cultures.53 For the preoperative tests, WBC was negative in 67/72 (93%) cases, with a negative polymorphonuclear percentage distribution in 64/70 (91%). ESR was negative in 36/42 (86%) cases that it was measured, and CRP was negative in 12/16 (75%) cases that it was obtained. Intraoperative histology was negative in 67/73 patients (92%). Of the 75 cases, the authors reviewed the most common organisms were present in 45/75 (60%), followed by S. epidermidis in 10/75 (13%). The mean time for the first growth of P. acnes was 5.1 days. In another recent study, Kelly and Hobgood reported on the rate of positive intraoperative cultures among patients without overt signs of infection, including negative intraoperative Gram stain and frozen section (less than five polymorphonuclear leukocytes per high-powered field), who underwent revision shoulder arthroplasty.30 Twenty-eight revision cases were identified, with 8 (29%) showing positive culture results. P. acnes grew in 6/8 (75%) positive cultures. The average time for cultures to grow an organism was 7 days, and the authors noted that cultures were held for 14 days at their institution to detect slower-growing organisms, such as P. acnes. On preoperative bloodwork, WBC was negative in 25/26 (96%) cases, polymorphonuclear cells were normal in 26/27 (96%), ESR was negative in 12/16 (75%), and CRP was negative in 7/12 (58%).
arthroplasty and had positive intraoperative cultures.53 For the preoperative tests, WBC was negative in 67/72 (93%) cases, with a negative polymorphonuclear percentage distribution in 64/70 (91%). ESR was negative in 36/42 (86%) cases that it was measured, and CRP was negative in 12/16 (75%) cases that it was obtained. Intraoperative histology was negative in 67/73 patients (92%). Of the 75 cases, the authors reviewed the most common organisms were present in 45/75 (60%), followed by S. epidermidis in 10/75 (13%). The mean time for the first growth of P. acnes was 5.1 days. In another recent study, Kelly and Hobgood reported on the rate of positive intraoperative cultures among patients without overt signs of infection, including negative intraoperative Gram stain and frozen section (less than five polymorphonuclear leukocytes per high-powered field), who underwent revision shoulder arthroplasty.30 Twenty-eight revision cases were identified, with 8 (29%) showing positive culture results. P. acnes grew in 6/8 (75%) positive cultures. The average time for cultures to grow an organism was 7 days, and the authors noted that cultures were held for 14 days at their institution to detect slower-growing organisms, such as P. acnes. On preoperative bloodwork, WBC was negative in 25/26 (96%) cases, polymorphonuclear cells were normal in 26/27 (96%), ESR was negative in 12/16 (75%), and CRP was negative in 7/12 (58%).
Current Diagnostic Recommendations
The American Academy of Orthopaedic Surgeons has recently approved its clinical practice guidelines for the diagnosis of periprosthetic joint infections of the hip and knee.14 These recommendations are based on the evaluation of the current best evidence available in the hip and knee literature through a series of systematic reviews, and may be useful as a starting point for guidelines in shoulder arthroplasty as well. The authors strongly recommend testing of both serum ESR and CRP in the evaluation for prosthetic infection, as the use of either test alone is less reliable than when both are combined.14 Prosthetic infection has been shown to be unlikely following a negative result on both tests, while suspicion for infection is raised and further work-up is needed if both tests are positive.14 Joint aspiration is strongly recommended if either ESR or CRP is elevated, with the synovial fluid sent for culture, WBC count, and differential WBC count. The authors recommend patients be off of antibiotics for a minimum of 2 weeks before an aspiration. If a patient is ultimately taken to surgery with a suspicion for infection, it is strongly recommended that multiple peri-implant tissue/fluid samples be obtained intraoperatively for culture and frozen section, with antibiotics withheld until these samples are obtained. Although the authors recommend obtaining intraoperative cultures, they strongly recommend against the use of intraoperative Gram stain as a method of ruling out prosthetic infection, based on the current evidence in the hip and knee literature.14
With much less high-quality data specific to shoulder arthroplasty available to guide decision-making, these recommendations from the hip and knee literature may be used as general guidelines when evaluating for possible prosthetic infection in the shoulder. However, further research is required to determine if modifications in these guidelines are needed. Certain criteria may be found to differ in the shoulder, particularly in the setting of infections with less virulent organisms that are common in shoulder arthroplasty.
TREATMENT
The goals of management of an infected shoulder prosthesis should include eradicating the infection, while maximizing pain relief and shoulder function.48 Treatment options have included antibiotic suppression alone, irrigation and debridement with implant retention, one-stage exchange with antibiotic-impregnated cement fixation, two-stage exchange with a temporary antibiotic-impregnated cement spacer, resection arthroplasty with or without permanent spacer placement, arthrodesis, and amputation.1,5,8,9,10 and 11,13,15,24,26,29,30,33,38,39,40 and 41,45,48,49,50,51,52 and 53,55,57 A precise treatment algorithm does not currently exist, but the results of preoperative testing, time since the index arthroplasty, the specific organism, patient comorbidities, the status of implant fixation, and the status of the deltoid and rotator cuff and glenoid and humeral bone stock should all be considered in determining the appropriate treatment.
Long-term antibiotic suppression is typically used in the case of a patient with medical comorbidities that make the risk of surgery unacceptably high, or in patients unwilling to have surgery. It may also be considered in certain situations when a patient has minimal or no pain, is not septic, and the isolated organism is of low virulence. There is little literature on outcomes of antibiotic suppression following infected shoulder arthroplasty. A recurrent infection rate of 60% was reported in one extremely small series.10 Outcomes as a treatment following infected total knee arthroplasty have been reported to be poor, with rates of successful suppression as low as 24%.21,47
Irrigation and debridement with implant retention is a possible treatment option with a stable prosthesis in early or acute infections (type 2), and in those that occur from an acute hematogenous event (type 3). Infection with a highly susceptible or low virulent organism in this setting may lend further support for this as a treatment option. Aggressive debridement of all concerning tissues should be performed with this technique, and humeral head exchange should also be considered if a modular system is implanted. Removal of the humeral head also allows increased exposure for the debridement procedure, particularly posteriorly. Following surgery, the patient has a peripherally inserted central catheter (PICC) line placed to receive long-term intravenous (IV) antibiotics. Typically, a 4 to 6 weeks course of organism-specific IV antibiotics is given. A period of oral antibiotics may then be administered following the completion of IV antibiotics, depending on the particular case and organism.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree