Lumbar Pseudarthrosis
S. Tim Yoon
Kai-Jow Tsai
OVERVIEW
For as long as surgeons have attempted to fuse the lumbar spine, pseudarthrosis of the lumbar spine has plagued them. Lumbar pseudarthrosis is defined by a failed union after fusion surgery, leading to the anatomical condition of continued significant motion of the spinal lumbar motion segment. This condition can lead to back pain, radicular pain, deformity, and hardware failure. Failure of fusion is associated with a significant chance of reoperation and has been shown to lead to poor long-term outcome (1). The cornerstones of lumbar fusion are bone grafting technique and immobilization of motion segments to be fused. Historically, fusion techniques relied on external immobilization, but modern techniques of internal fixation have increased spinal fusion rates. Nevertheless, pseudarthrosis still occurs at a significant rate. This chapter will discuss the incidence of pseudarthroses, the anatomical considerations related to pseudarthroses, and methods of preventing and managing pseudarthroses, as well as present some typical case examples of this complication.
INCIDENCE
Posterior arthrodesis of the spine for progressive deformity first was introduced by Hibbs in 1911. Indications for lumbar arthrodesis have expanded beyond deformity (scoliosis, kyphosis, etc.) and now include degenerative conditions such as intervertebral disc degeneration and segmental instability, as well as trauma, infections, and tumor reconstruction (2,3). Factors that influence pseudarthrosis rates depend on surgery-related factors and patient-related factors. Single-level posterior spinal fusion yields pseudarthrosis rates that ranges from 0% to 60% (4,5,6,7,8,9,10,11,12,13). Patient-related factors are also important. The risk of pseudarthrosis may be as low as 1% to 2 % in treating adolescent idiopathic scoliosis, whereas in the adult population, a risk of 25% to 30% has been reported with instrumented, posterolateral lumbar fusion (14). It may be as high as 45% when a thoracolumbar fusion is extended to the sacrum (14).
The wide range of reported pseudarthrosis rates reflects the differences in surgical techniques, indications, patient population, and the criteria for pseudarthrosis. In analyzing the literature, it is very important to identify the criteria for determining spinal fusion. Clinical criteria for diagnosis of pseudarthrosis are the least sensitive. The addition of static radiographs is more helpful, and dynamic radiographs improve the sensitivity even further. The addition of increasingly sophisticated imaging modalities, such as CT scanning, improves the detection of pseudarthrosis even further (8). Some newer well-controlled studies have indicated a relatively high rate of nonunion (4,6,8). This may be a reflection of the strict radiographic criteria of these newer studies.
CLINICAL PRESENTATION
Making an accurate diagnosis of lumbar pseudarthrosis of the spine is difficult because there is no pathognomonic clinical symptom or sign and no single accepted imaging criterion. Clinical presentation can be misleading, since as many as 50% of patients have been reported to be asymptomatic (4,6). However, clinical symptoms such as mechanical low back pain, progression of stenosis at the fusion level, and development of deformity can suggest a pseudarthrosis. Significant pain and disability have been documented in patients with pseudarthrosis after failed surgical fusion attempts (15,16,17,18,19,20). Confirmation with imaging is usually required to make the diagnosis. However, currently there is no diagnostic imaging modality that can detect pseudarthrosis with 100% accuracy. Surgical exploration is therefore the current “gold standard” in diagnosing pseudarthrosis (21).
Clinical presentation of pseudarthrosis is typically a history of initial postoperative improvement of low back pain, followed by return of low back pain. Early on, there may be implant loosening, as noted by halos at the bone implant interface or by implant failures that typically happen later in the postoperative course. A mechanical component to the patient’s pain, such as excellent rest relief and exacerbation of low back pain with physical activity, can be helpful to make the diagnosis of mechanical etiology for the pain.
In patients with suspected pseudarthrosis, it is important to consider coexisting infection. A history of previous wound infection can be a clue. Laboratory workups with blood C-reactive protein level and erythrocyte sedimentation rate help with this assessment. Glassman et al. reported 24% (5 out of 19) of postoperative infection cases developed pseudarthrosis at 1 year follow-up (22). Some of the infections are obscure. Schofferman et al. reported that two of seven patients had a positive culture with low virulence diptheroid with normal ESR results (23). Patients who are leukopenic with absolute lymphocyte counts under 1,200 and patients who are malnourished are at greater risk for an occult infection.
PLAIN RADIOGRAPHY
Anterior-posterior (AP) and lateral radiographs should be taken at periodic intervals after spinal fusion surgery to monitor the progression of the fusion mass and instrumentation. The bone graft typically becomes remodeled into a more flowing bony mass, and a clear pattern of continuous bone from one vertebra to the other suggests a union. In contrast, a clear and persistent radiographic cleft at 12 to 18 months after fusion surgery should be considered a pseudarthrosis. However, the absence of a radiographic cleft does not exclude the presence of a pseudarthrosis, as this criterion has a significant false negative rate. Kant et al. demonstrated that there is only a 68% accuracy using the presence of continuous trabecular bone on radiographs when compared to pseudarthrosis assessment by surgical exploration (24). In Kant et al.’s report, the L4-L5 level was the most difficult level to fuse and the most difficult to interpret using x-rays. Practically speaking, however, L5-S1 is an area that is probably more difficult to image with plain radiographs. Albert et al. reported a higher success rate (32 of 39 cases, 82%) with using plain radiography to diagnosis pseudarthrosis (25). Besides the presence of a cleft in the fusion mass, other radiographic signs of pseudarthrosis include failed incorporation of bone graft, progressive resorption of bone graft, broken spinal implants, loosening of bone-implant interface, and progression of deformity (26).
Flexion and extension lateral lumbar radiographs have been an integral part of assessing fusion (27,28,29). Interbody fusion success was determined by (a) the absence of motion on flexion-extension radiographic views, using a radiographic overlay method to assess angular change at the segment(s); (b) the absence of halo around a cage, on AP, Ferguson, and lateral radiographic views; and (c) the continued presence of visible bone within each cage, as seen on the Ferguson radiographic view. There is some controversy about the amount of angular change between the vertebrae that is consistent with spinal fusion. In the U.S. Food and Drug Administration’s (FDA) Investigational Device Exemption (IDE) trial of the Bagby and Kuslich (BAK) cage, angular change of greater than 7 degrees was thought to indicate nonunion, and less than 3 degrees of motion indicated union; 3 to 7 degrees was an intermediate area where case-by case judgment was required (29). However, many surgeons now feel that this criterion is too permissive and that any definitive motion seen on x-rays should be suspected of being a pseudarthrosis. While the usefulness of flexion and extension views to evaluate fusion status is well-established for the cervical spine, its usefulness in the lumbar spine has been questioned (30,31). This may be due to the difficulty in precisely measuring the translatory or angular motion due to variability in radiographic imaging. Other techniques such as radiostereometrics have been developed to improve the radiographic assessment (32,33), but they have not yet been widely adopted in the literature.
COMPUTED TOMOGRAPHY (CT)
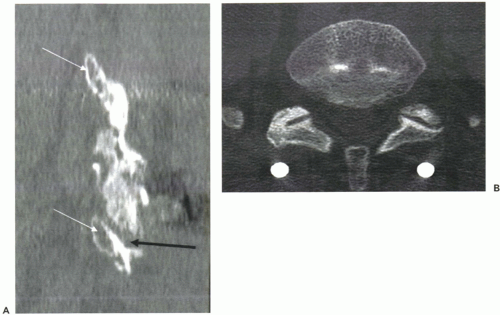
Because plain radiographic evaluation has certain limitations, CT scans are being used more often. Typically, 6- and 12-month CT scan evaluations can help make a more definitive determination of the status of the spinal fusion. The authors prefer to use 1-mm contiguous cuts using a spiral CT scanner, obtaining reconstructed views in the sagittal and coronal view. For posterolateral fusion, excellent visualization can be obtained in the intertransverse regions with sagittal (Fig. 14.1A) and coronal reconstructed views. Axial views are usually better at assessing facet fusion (Fig. 14.1B). The combination of reconstructed views and axial views provides an excellent assessment of fusion status and can clearly demonstrate a pseudarthrosis where plain radiographs would indicate a solid fusion. Three-dimensional surface reconstructions have not been as helpful, as there can be significant volume averaging, and visualizing the surface is better done on a powerful computer workstation. Metal instrumentation can sometimes obscure the details of imaging; however, titanium implants usually produce relatively little scatter artifact. Because of this, titanium implants are often preferred over stainless steel implants in degenerative spinal surgeries.
BONE SCAN
Bone scintigraphy with single photon emission computed tomography (SPECT) has been evaluated for efficacy in
identifying pseudarthrosis in patients with persistent back pain after fusion surgery. In the fusion mass of a successful fusion, SPECT images are characterized by a diffusely increased uptake of radiotracer. In contrast, focally increased uptake has been shown to be related to bony pseudarthrosis (31,34,35). Albert et al. evaluated SPECT scanning in 38 patients, in whom SPECT correctly identified 7 of the 14 pseudarthrosis and 14 of 24 solid fusions, with a sensitivity of 0.50 and specificity of 0.58 (34). They did not recommend SPECT scanning as a routine modality for use in the diagnosis of pseudarthrosis. Larsen et al. have compared plain flexion-extension film, computed tomography, and bone scintigraphy in 25 patients with previous lumbar fusion with pedicle screw instrumentation and persistent complaints of severe low back pain. Larsen et al. used surgical inspection, which was taken as absolute evidence of fusion or pseudarthrosis (31,35). They found bone scintigraphy sensitivity to be poor.
identifying pseudarthrosis in patients with persistent back pain after fusion surgery. In the fusion mass of a successful fusion, SPECT images are characterized by a diffusely increased uptake of radiotracer. In contrast, focally increased uptake has been shown to be related to bony pseudarthrosis (31,34,35). Albert et al. evaluated SPECT scanning in 38 patients, in whom SPECT correctly identified 7 of the 14 pseudarthrosis and 14 of 24 solid fusions, with a sensitivity of 0.50 and specificity of 0.58 (34). They did not recommend SPECT scanning as a routine modality for use in the diagnosis of pseudarthrosis. Larsen et al. have compared plain flexion-extension film, computed tomography, and bone scintigraphy in 25 patients with previous lumbar fusion with pedicle screw instrumentation and persistent complaints of severe low back pain. Larsen et al. used surgical inspection, which was taken as absolute evidence of fusion or pseudarthrosis (31,35). They found bone scintigraphy sensitivity to be poor.
MAGNETIC RESONANCE IMAGING (MRI)
MRIs can be of some limited utility in determining fusion status. MRI patterns of high signal intensity changes in the subchondral marrow of adjacent fused vertebrae on T1-weighted images and low signal intensity changes on T2-weighted images indicates fusion (31). In contrast, MRI patterns of low signal intensity on T1-weighted images and high signal intensity on T2-weighted images seen in subchondral vertebral bands indicate pseudarthrosis (31). The artifact created by metallic implants may be reduced by fast spin-echo sequences. In general, MRIs are less important in assessing fusion status than in identifying spinal stenosis or other soft-tissue pathology that is not as well seen on radiographs or CT scans.
RELEVANT ANATOMY
The size of the fusion bed has a significant influence on fusion rates. In general, the larger the fusion bed, the less difficult it is to achieve solid fusion. Molinari et al. showed that L5 transverse process surface of less than 2 cm2 was correlated with higher rates of pseudarthrosis when trying to fuse L5-S1 (9). In contrast, the closer together the decorticated regions to be fused are, the less difficult it is to achieve solid fusion. The anatomy of the interbody is favorable for fusion because the fusion surfaces are relatively close together and the surface area available for fusion is large in comparison to the distance to be bridged by new bone. Perhaps because of this, lumber interbody fusion rates have been in the high 90% range in recent clinical trials (36,37). In contrast, intertransverse process fusion is more difficult than interbody fusion. This may be because the transverse processes are farther apart than vertebral end plates, fusion surface areas are smaller, and soft-tissue interposition across the transverse process can prevent successful fusion.
Biomechanical stability of the segments to be fused has a significant effect on fusion success. The stiffer the segment, the more likely the segment is to fuse, and the less effort surgeons have to exert on immobilizing the segment, In some situations, segments are so severely degenerated and stiff that they are well on their way to autofusion even without surgical intervention. In contrast, some segments are much less stable and require more attention to mechanical stabilization. The less-stable segments arise due to many different causes, such as surgical destabilization, trauma, infection, or congenital defects. In this situation, combined anterior and posterior surgery may be required to achieve the stiffest mechanical construct (38,39). Another factor to consider is the overall mechanical forces on the fusion mass, with tension producing a smaller fusion mass and higher pseudarthrosis versus compression producing a more robust fusion mass and higher fusion rates. Therefore, surgeons may have to correct the global and local alignments to achieve the highest possible fusion rates.
Fusion at the lumbosacral junction can be difficult, especially when there is a long fusion above it. This is related to the increased mechanical stress with the long lever arm above Lt-S1, and also the difficulty with sacral fixation. Therefore, long fusions that terminate at the sacrum should be approached with a thought toward increasing the mechanical stability of the construct. Four screws in the sacrum (S1 and S2, either pedicle or alar screws) have met with success. Another approach is to use iliac fixation. Interbody fusion either as a posterior lumbar interbody fusion (PLIF), transforaminal lumbar interbody fusion (TLIF), or anterior lumbar interbody fusion (ALIF) should be considered.
ETIOLOGY AND PREVENTION OF PSEUDARTHROSIS
The factors that increase the difficulty in achieving a solid fusion after surgery can be grouped into those related to the patient and those related to the surgery. These factors can be represented graphically in what could be called a “difficulty continuum,” achieved by combining both patient-related factors (x-axis) and surgery-related factors (y-axis) (Fig. 14.2). Clearly the young, healthy patient undergoing a single-level interbody fusion has a much higher fusion rate than an elderly smoker on steroids undergoing multilevel posterolateral fusion. Between these two extreme examples, many different levels of difficulty exist, forming a difficulty continuum for spinal fusion. By recognizing this continuum, it becomes clear that no single surgical technique is ideal for all situations and that each case should be evaluated individually. Given that there are so many different combinations of factors that affect fusion rate, surgeons do not have the luxury of being able to quote a high-quality, prospective study for each surgical scenario. Therefore, surgeons have to rely on broad principles to customize the surgery, factoring in the magnitude of the surgery, the constitution of the patient, and the overall clinical goal for each individual patient.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree