Lower Leg and Ankle
Neeru Jayanthi
Lower Leg
The lower leg—that portion of the body between the knee and ankle but not inclusive of those two joints—is the site of frequent injury. Injuries to the lower leg make up 3% to 5% of all reported sports injuries. If only those sports in which running is a major component are counted, the injury rate increases to 30% of all injuries.
Anatomy
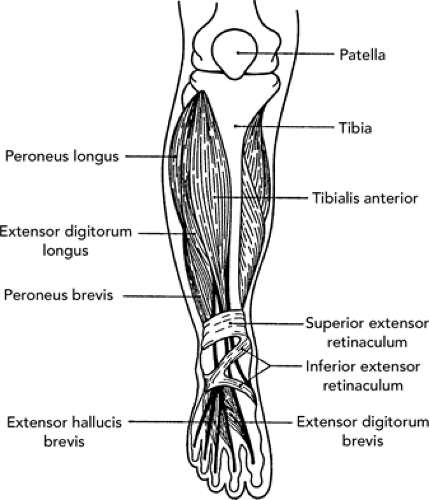
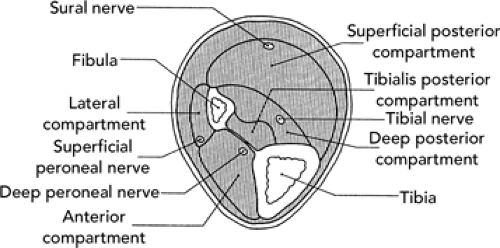
The proximal lower leg serves as the insertion for muscles originating from the thigh. The distal portion of the lower leg is intimately involved in the function of the ankle joint. The cortical surfaces of the tibia and fibula are the origin of muscles that move the ankle, foot, and toes (see Figure 32.1). The tibia is the main weight-bearing bone of the lower leg. It is triangular in shape, with its anterior border and anterior medial surface primarily subcutaneous. The fibula is situated posterolaterally in the lower leg. Although not directly involved with the knee joint, it does form the distal attachment of the lateral collateral ligament (LCL). In addition, it forms the vital lateral border of the ankle mortise. Connecting the tibia and fibula is an interosseous membrane that serves as the floor of the anterior compartment of the leg. On cross-section, the lower leg (see Figure 32.2) consists of four muscular compartments separated by intermuscular septa or fascial sheaths and possibly another subcompartment. The anterior and lateral compartments permit little flexibility or expansion of the muscles contained within. Conversely, the posterior compartment is a loosely contained space not subject to the same constriction that might result from expansion due to injury. The muscles and neuromuscular bundles of the various compartments are as follows:
Anterior compartment. This consists of tibialis anterior, extensor digitorum longus, extensor hallicus longus, and peroneus tertius muscles; deep peroneal nerve.
Lateral compartment. This consists of peroneus longus and peroneus brevis muscles; superficial peroneal nerve.
Posterior compartment. This compartment is subdivided into the following:
superficial posterior—gastrocnemius, soleus and plantaris muscles, sural nerve.
deep posterior—flexor digitorum longus and flexor hallicus longus muscles, posterior tibial vessels and nerves (including the peroneal vessels)
subcompartments of deep posterior—fibular origin of flexor digitorum longus muscle, sometimes tibialis posterior muscle
Contusions
Contusions of the lower leg are extremely common because of the exposed anatomy of the lower leg. Sports in which contusions occur regularly include the following:
Soccer, rugby, football—blow from an opponent’s kick, usually to anterior lower leg
Field hockey—blow from an opponent’s stick to the anterior or posterior lower leg
Baseball—two common mechanisms are a blow from a batted ball or laceration and contusion resulting from being “spiked” by a sliding opponent
Biomechanics. Acute trauma to the exposed lower leg usually occurs anteriorly and results in a contusion and/or hematoma of the underlying structures. These injuries are treated as bruises.
Signs and symptoms. Pain at impact, disability secondary to guarding, and swelling are associated with most soft tissue trauma. Swelling comes from extravasation of blood. Subsequent discoloration of the skin progresses within hours of injury. Assessment should
include evaluation of the neurovascular bundles examined distally, as well as muscle group testing using active and passive resistance.
Differential diagnosis. Although a contusion to the lower leg is considered a straightforward injury, several complications can develop. Consider these specific injuries in the differential diagnosis:
Subperiosteal hematoma. Commonly seen in the lower leg because of the subcutaneous nature of the tibia. This injury is suspected when the degree of debilitation and pain is severe and out of proportion to the relatively negative physical examination. X-rays are necessary to confirm the diagnosis and reveal periosteal elevation and/or thickening. Magnetic resonance imaging (MRI) can demonstrate edema in the bone and any hematoma formation with reasonable accuracy. Initial treatment includes close observation and continued ice therapy, long after the normal contusion treatment guidelines.
Tibial or fibular fracture. Fractures as a result of blunt trauma are unusual, but should be suspected when considering a diagnosis of a subperiosteal hematoma (see preceding text). Pain is elicited on palpation over the fracture. In addition, percussion at the ankle can produce pain over the fracture site. Most of these fractures involve the weight-bearing tibia and usually prevent the athlete from walking. If the history of trauma is to the lateral side of the leg, fracture to the fibula should be suspected. Examination of the fibula is difficult except at its most proximal and distal margins, so a fracture should be considered if palpation at these margins produces pain anywhere along the fibula. Remember, as a relatively non–weight-bearing bone, the fractured fibula will allow ambulation without much pain. If pain is present, the fibular fracture is usually more proximal in nature as a result of muscle spasm along the insertion of the upper leg muscles. Special note—a proximal fibular fracture can also mimic laxity in the LCL.
Hematomas. These usually occur in the anterior and lateral compartments and are present in most contusions of the lower leg. A severe hematoma in the anterior or lateral compartment may rarely lead to a compartment syndrome and eventual ischemia or necrosis of muscles, if not watched and controlled. Although uncommon, a hematoma to the posterior calf can cause damage to the venous outflow system and cause stasis of blood flow and possibly even a deep vein thrombophlebitis. Suspect such a complication 36 to 48 hours following an injury if an athlete complains of increasing dull ache in the posterior calf aggravated by ambulation or attempted exercise. Signs include observation of a red streak proximal to the tender calf (lymphangitis) and palpation of a tender “cord” (thrombosed vein). The classic “Homan’s sign” has not been proven to be a reliable predictor of deep venous thrombosis (DVT). Treatment of DVT includes bedrest, elevation, and anticoagulation.
Peroneal nerve palsy. This injury is secondary to direct trauma of the peroneal nerve as it courses superficially over the proximal lateral surface of the lower leg, usually at the point of the head of the fibula. The injury will vary greatly according to its severity, but usually results in a transient period of pain, numbness, and paresthesia in the distribution of the common peroneal nerve. The patient usually describes a sharp shock-like pain that shoots to the lateral side of the leg and foot.
Most of these cases need no specific treatment except for control of the resultant contusion. The presence of continued neurapraxia should eliminate the athlete from competition. Presence of a foot drop should be an indication for further investigation such as electromyography (EMG) and nerve conduction studies, as well as possible referral. There are multiple case reports of peroneal nerve palsy induced by nontraumatic situations including ice application to the lateral knee due to its relative lack of insulation in this region (1). It is important to remember this point when treating lateral knee injuries.
Treatment. Most minor contusions and/or hematomas are treated with ice, elevation, and judicious use of nonsteroidal anti-inflammatory drugs (NSAIDs) to control inflammation and prevent serious complications. Rest and reduced or limited weight-bearing should be advised if the contusion limits or affects the function of the leg. This may prevent further injury or extension of injury.
Return to play. With the resumption of the full function of the leg, an athlete can return to play so long as there is protective padding over the injured area. Rarely, aspiration of fluid from the hematoma can be considered in the acute setting, because of its inherent risks. Laceration, hematoma, and contusion (such as those seen in baseball injuries) should be appropriately cleaned, repaired, and sometimes treated with prophylactic antibiotics for a 1-week period. Any breakage of skin should be accompanied by tetanus prophylaxis unless immunization is up-to-date (within 10 years for adults; within 5 years for children).
Prevention. Sports in which lower leg injury is a common threat, have adopted standard equipments for their prevention (shin guards in soccer, ice hockey, and for baseball catchers). A patient with a past history of serious injury should be protected by a rigid shin guard.
Medial Tibial Stress Syndrome
Medial tibial stress syndrome (MTSS), sometimes referred to as shin splints is typically painful at the posteromedial border of the tibia generally because of an increase in the volume of impact exercises. Running generates many of these injuries and shin splints make up 15% of all running injuries, whereas stress fractures of the lower leg accounted for another 15%. Rapid increase in training regimens by either intensity, duration, or frequency, as well as running more than 40 miles per week and for 7 days a week are all considered as risk factors for the development of this type of overuse injury (2). Theories regarding the pathogenesis include a periostitis of the posteromedial tibia as well as a traction injury of the attachment of the tibialis posterior, flexor digitorum longus, or soleus muscles to the tibia.
Biomechanics and etiology. MTSS results from any number of the following factors. Poor conditioning, inappropriate training, improper footwear, running on inconsistent surfaces, running on sloped or banked surfaces, running on unbanked tracks, or any situation that allow for excessive foot pronation or excessive external rotation of the hip. Malalignment such as increased navicular drop and pronated foot or tibial varus may place undue stress on the posteromedial muscle-bone interface. This stress takes place at midstance with the foot pronated.
Diagnostic aids. After 3 to 4 weeks, x-rays may show irregularity of the bony cortex and new bone formation along a portion of the wide attachment of the tibialis posterior tendon to the proximal midshaft of the fibula and tibia. Bone scans may reveal increased uptake in a longitudinal pattern in the same area when compared with the localized, usually transverse, uptake seen in a stress fracture.
Treatment. In addition to treatment, in accordance with grades 2 and 3 of the overuse guidelines, proper support and possible orthotic devices should be considered to prevent hyperpronation and excessive tibial rotation. Some trainers will attempt taping to reduce the traction forces, as well as modalities for symptomatic relief.
Prevention. The best way to treat MTSS, like many overuse injuries, is to prevent it from happening. Some suggestions include careful training programs not exceeding 40 miles per week, and at least one rest day each week. A significant taper is necessary prior to distance events for injury prevention as well as for performance. Advanced runners will be careful to use running shoes with a good midsole cushion, and even consider alternating two pairs of the same type of shoes, to allow the return to normal cushion size on “off days”. Also limiting mileage of one pair of shoes to 200 to 400 miles is advisable.
Stress Fractures
Epidemiology. Stress fractures occur most often in the lower leg, usually from a rapid or significant change in the volume of running or jumping activities, or because other factors are overloading the tibia and less commonly the fibula. Location varies but most of them occur in the distal third of the tibia, and occasionally in more high-risk areas such as the proximal tibia, anterior tibial cortex or medial malleolus. Less common proximal and distal fibular stress fractures also occur. Prognostically, stress fractures occurring proximally may be a greater cause for concern. Anterior tibial cortex stress fractures are notorious for protracted healing and progression to nonunion. Other high-risk stress fractures include the femoral neck (particularly tension-sided), patella, fifth metatarsal, tarsal navicular, talus, and
first toe sesamoid. Early recognition of these stress fractures that have a propensity for delayed or nonunion may help prevent some long-term complications.
Biomechanics and etiology. Lower extremity stress fractures have many of the same predisposing risk factors as MTSS, which was discussed earlier. However, these stress fractures typically involve an abnormal balance between osteoblast and osteoclast activity. Osteoclasts, which aid in bony breakdown, may have increased activity in a stress reaction. Without appropriate rest to allow reduction in overload to the bone, a stress fracture may occur. Use of ankle braces may increase the risk of fibular stress fractures. Footwear required for particular sports (e.g., the rigid boots worn by figure skaters) may also increase the risk of fibular stress fracture.
Signs and symptoms. Consistent with Grade 4 overuse. The primary complaint is pain that gets worse with activity and initially relieved by rest. Later in the process, the athlete may complain of pain due to normal daily activities. They may also complain of swelling in the area of the stress fracture and may have painful limp.
Differential diagnosis.
MTSS—pain consistent with Grade 2 or 3 overuse.
Exertional compartment syndrome—pain worsens as exercise continues, but decreases with cessation of activity.
Popliteal artery entrapment syndrome—immediate pain with exercise, followed by numbing and tingling.
Peroneal nerve entrapment.
Tenosynovitis of the dorsiflexors of the foot—superficial pain located directly over the dorsal-anterior portion of the lower leg with elicitation of pain on dorsiflexion.
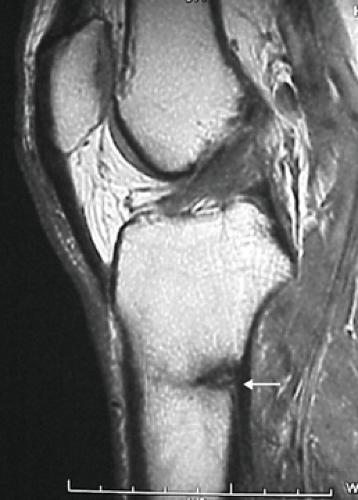
Diagnostic aids. A complete tibia/fibula x-ray can be helpful but may be effective only after at least 3 to 4 weeks of symptoms. Periosteal bony reaction or sclerosis may be seen in the area of tenderness. Anterior cortex tibial stress fractures will have the “dreaded black line” and will often need clarification with a computed tomography (CT) scan. Bone scans can be ordered for confirmation of a diagnosis, which is often important, because (except for the cases in which a fracture line is seen) one cannot determine the age or metabolic activity of an injury based on x-ray changes alone, that is, the changes seen on x-ray may be due to an old injury and may not be the cause of current pain. MRI may demonstrate stress fractures well with edema on T2 weighted images and low intensity signal on T1 or proton density images (see Figure 32.3), but often is not cost-effective. Limited MRI has become a diagnostic option in some regions utilizing primarily short tau inversion recovery (STIR) images to evaluate edema patterns.
Initial treatment. A significant reduction in impact volume is often adequate to treat straightforward distal tibia stress fractures in approximately 4 to 6 weeks. Higher-level athletes and those who are interested in earlier return to activity may be interested in pneumatic walking boots. Leg length ankle stirrup braces are available, and may allow for earlier low-impact activities for athletes with tibial stress fractures. To maintain conditioning, alternative non–weight-bearing sports such as swimming, running in water, or bicycling are appropriate. Once the individual is able to walk without pain, light intensity training (LIT) guidelines are applied. Return to activity should follow gradual increase in intensity, duration, and frequency, as well as sport-specific functional progression. Fibular stress fractures may often be treated similarly to distal tibia stress fractures, and also returned to activity in a graded manner.
Proximal tibial stress fractures and medial malleolus stress fractures usually require longer periods of immobilization. In medial malleolus stress fractures, Shelbourne et al. recommend internal fixation if a fracture line is present (3).
Anterior tibial cortex stress fractures have a poor prognosis, and will need a combination of rest, non–weight bearing and immobilization for at least 4 to 6 months. Use of a pneumatic lower leg brace and modified rest allowed return to unrestricted activity at an average of 12 months from presentation (4). None of the four patients in the study required surgery. Rest
and pulsed electromagnetic therapy for at least 3 to 6 months has also been shown to return athletes to competition at an average of 8.7 months of treatment and 12.5 months from initial symptoms (5). In this study, only one of eight patients needed bone grafting procedure, whereas all of them demonstrated complete healing. If healing is not achieved, intramedullary rod placement in the tibial shaft should be considered. In one series, 9 of 17 patients failed conservative treatment at 6 months, progressed to delayed nonunion and required intramedullary rod placement (6). All five patients had good or excellent results after intramedullary nailing in patients who failed nonoperative therapy for more than 1 year (7).
Prevention. Although it would seem that certain people are prone to develop stress fractures, such a hypothesis has not been consistently proven. Many of the prevention studies discussed with MTSS apply with lower extremity stress fractures as well. Repeated stress fractures are certainly a cause of concern and causes of osteopenia/osteoporosis should be considered, particularly in the female athlete. Prolonged amenorrhea secondary to hypoestrogenism may be a factor. Dietary (low calcium and/or vitamin D intake) and eating pattern (anorexia nervosa and bulimia nervosa) problems may also play a role. Careful clinical monitoring of the progression from overuse injury to stress fracture in athletes should be done by medical personnel who are comfortable in evaluating such injuries. The most important preventative measure may simply be adopting a “start low, go slow” attitude to changes in training regimens.
Compartment Syndrome
Epidemiology. Theoretically, the signs and symptoms that make up a compartment syndrome could happen in any of the four major muscle compartments located in the lower leg. The most commonly involved compartment is the anterior compartment. Next in order of frequency is the lateral compartment.
Biomechanics. These syndromes can be either acute (trauma) or more commonly chronic (overuse), and are the result of intrinsic swelling followed by compression of vascular and muscular structures. The initial swelling is the result of overload on the muscles comprising the compartment.
Symptoms. The onset of pain over the compartment begins within the first 5 to 10 minutes of exercise, and is usually relieved when the activity ceases. This differentiates compartment involvement from that of generalized overuse (MTSS), where pain usually begins later during the exercise and may continue well after exercise. Numbing or tingling of the distal lower extremity may be present and reflects involvement of the neurovascular bundle, but this is often a late finding. There is controversy surrounding the deep posterior compartment incorporating the flexor muscles of the foot and the posterior tibial neurovascular bundle.
Signs. In the ambulatory setting, swelling and possible edema of the compartment may be noted. Compartment musculature will be painful to touch, possibly weak, but seldom is neurovascular compromise clinically evident. Passive stretching of the compartment muscles will cause pain. Clinical signs are usually historical, and it may be worthwhile to have the athlete formally note the timing of his/her symptoms with exercise.
Diagnostic aids. Compartment testing is the gold standard for diagnosis of this condition. Normal resting compartment pressures are 0 to 8 mmHg. Exertional compartment syndrome can be diagnosed with resting compartment pressure greater than 15 mmHg; 1-minute postexercise pressure greater than 30 mmHg; or 5-minute postexercise pressure greater than 20 mmHg (8). This test should only be performed by physicians who are experienced and comfortable with the procedure because of the possible risks involved, which includes inducing acute compartment syndrome.
Differential diagnosis. In addition to MTSS and tibial/fibular stress fracture, consider thrombophlebitis, osteomyelitis, cellulitis, tumor, nerve entrapment, and intermittent claudication.
Treatment. Correction of biomechanics, especially those mechanics that isolate the muscle group(s) involved. In the case of chronic exertional compartment syndrome, rest, stretching exercises, massage, and ice may be helpful. Because of the risk of formal compartment pressure testing, these conservative treatments may be instituted when the diagnosis is suspected. If these treatments fail, formal testing should be considered. Fasciotomy is required in the acute setting and is often required because of failure of conservative measures in the chronic exertional setting (9).
Medial Gastrocnemius Strain
Anatomy. The gastrocnemius (from the superficial posterior compartment) is injured most often in its medial belly when the foot is dorsiflexed and the knees are forcibly extended in an eccentric manner. When this happens, the medial head of the gastrocnemius near its musculotendinous junction can strain or tear. The gastrocnemius is vulnerable because it works across two joints and is a muscle of short action.
Epidemiology. The middle-aged athlete (usually between 35 and 45 years of age) appears most at risk. Racquet sports are most commonly implicated so that this condition is sometimes termed tennis leg.
Treatment. Following the standard RICE (rest, ice, compression, and elevation) regimen along with passive
stretching exercises are done initially. Ultrasound can be used but should be avoided if a large hematoma is suspected as the deep heat generated may induce further bleeding. Active stretching and strengthening exercises can usually be started within a week. A calf sleeve may provide some warmth to the area to facilitate stretching. If a partial tear has taken place, a brief period of immobilization may be considered along with RICE protocol. A night splint to keep a passive stretch on the gastrocnemius during sleep may be very helpful initially as well. Advancement to active rehabilitation may take 1 to 2 weeks, even longer for higher-grade injuries. The prognosis for injuries to this muscle is good if treated early and appropriately.
Achilles Tendon Injuries
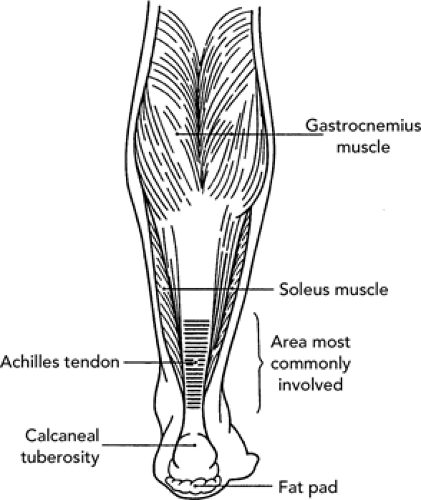
Anatomy. The Achilles tendon represents the conjoined tendon of the gastrocnemius and soleus muscles, the latter a major contributor to the plantar flexion strength of the foot. The extreme distal portion of the Achilles tendon appears to have a poor blood supply, especially in the region 2 to 6 cm above the insertion on the calcaneus. A normal tendon is extremely strong and able to withstand forces up to 2,000 pounds during fast running.
Epidemiology. Three clinical entities will be discussed here:
Achilles tendinitis—usually seen in repetitive overload overuse, common in running.
Complete tear or Achilles tendon rupture—seen in sports such as basketball, tennis, or skiing that involve sudden maximal contraction of the musculotendinous unit.
Partial tear—represents a crossover variant, with etiology representing either overuse and/or acute overload.
Achilles Tendinitis
This can be divided into two clinical presentations:
Acute—involving just the peritenon (tendon sheath), not the tendon itself. The peritenon is not a true synovial sheath as seen with other tendons in the body.
Chronic—the result of prolonged mucoid degeneration of the tendon substance itself. In the chronic condition, active inflammation may not be present, leading to the term Achilles tendinosis.
Biomechanics. The usual mechanism of injury is chronic, repetitive overload on the musculotendinous unit (see Figure 32.4). Biomechanical risk factors include tibia varus, tight hamstring muscles, tight calf muscles, and cavus foot. In addition, several training errors can cause this injury: constant hill running, shoes with rigid soles or soft heel counter, shifting from high heel dress shoes to low heel training shoes, changing from cross country running on uneven surfaces to more consistent elastic track surfaces, “ankling” too much in cycling. Repetitive eccentric loading of the Achilles tendon through jumping or running may contribute to injury of the tendon. Each of these situations results in increased pull and tension on the Achilles. Furthermore, the presence of other injuries such as plantar fascitis can cause the foot to land in excessive supination causing the ankle to dorsiflex to avoid pronating the foot. The Achilles tendon can move laterally or medially in response to such running biomechanics.
Signs and symptoms. As with any soft tissue overuse injury, signs and symptoms are consistent with those outlined in the earlier discussion of overuse syndromes.
Differential diagnosis. Although rarely a problem in running, partial rupture of the Achilles tendon should be ruled out.
Diagnostic aids. The work-up of severe or chronic tendinitis, especially if the examination has been accompanied by palpation and thickening of the Achilles tendon, may include x-ray evaluation of Kager’s triangle (seen in the lateral view of the affected ankle). This triangle is bound anteriorly by the flexor tendons of the foot, posteriorly by the Achilles tendon unit, and inferiorly by the os calcis. The radiolucent area will become more dense and less radiolucent in cases of severe tendinitis or partial rupture. Failure of conservative treatment of chronic Achilles tendonitis after a dedicated eccentric load tendon strengthening program for typically 6 months or longer may necessitate further imaging, and possibly surgical referral. In these less common, recalcitrant cases, ultrasound images may demonstrate tendon
pathology well. MRI can also be helpful in identifying abnormal Achilles tendon pathology, or particularly if there are other associated injuries.
Treatment. Initial treatment should consist of ice, initial stretching before exercise, use of NSAIDs, decreased mileage, avoiding banked roads and hills, and reassessing shoes, making sure that the following exist: flexible sole, molded heel pad. A heel lift at least 1/2 inch high has been shown to reduce the relative stress on the Achilles tendon, that is, the microtrauma throughout the day. It is imperative that this be done in conjunction with aggressive heel cord stretches, as wearing the heel lift functionally shortens the Achilles tendon. A walking-boot or ankle–foot orthoses (AFO) may be considered for temporary unloading of the tendon, in more severe cases. For recalcitrant cases, surgical debridement is rarely necessary. Ultrasound has some anecdotal success in these injuries. Rehabilitation should consist of stretching and eccentric strengthening exercises for the Achilles tendon. Eccentric tendon strengthening can show structural improvement of the tendon as well as clinical improvement within 3 to 6 months.
Prevention. Address any preexisting injury or deformity and attempt to correct before resuming exercise.
Special considerations. At no point in the therapy of Achilles tendinitis should steroid injections be used. The potential compromise to the vascular system of the Achilles tendon, as well as the weakening and possible necrosis stimulated by such an injection precludes this form of therapy for this injury.
Achilles Tendon Rupture
Epidemiology. Rupture occurs predominantly in male athletes in their third to fifth decade of life, in sports of sudden extreme movement such as basketball, tennis, long jumping, and skiing. Most ruptures occur 2 to 6 cm above the insertion of the Achilles tendon on the os calcis in the area of decreased vascularity. The left Achilles tendon is ruptured significantly more often than the right. Predisposing factors: (a) nonspecific degeneration perhaps secondary to vascular impairment produced by the particular repetitious form of exercise; (b) history of corticosteroid injections into the Achilles tendon; (c) repeated subclinical injury leading to necrosis and weakening of the tendon unit, and (d) normal tendon physiology that has undergone extreme pathomechanical stress.
Symptoms. Most published theories on acute tendon rupture include the statement that misdiagnoses can be as high as 20% to 25%. Usual symptoms include a sudden forced movement followed by a loud audible snap. The athlete will classically feel as if he/she has been struck on the back of his/her calf. Often, the initial pain will diminish and walking is possible. There is a weakness in plantar flexion and, at times, a feeling of the foot penetrating through the floor as if in plantar flexion.