Abstract
Spatial neglect (SN) is commonly associated with poor functional outcome. Adaptation to a rightward optical deviation of vision has been shown to benefit to SN rehabilitation. The neurophysiological foundations and the optimal modalities of prism adaptation (PA) therapy however remain to be validated. This study is aimed at exploring the long-term sensory-motor, cognitive and functional effects produced by weekly PA sessions over a period of four weeks. A double-blind, monocentric randomized and controlled trial (RCT) was carried out. Twenty patients with left SN secondary to stroke were included, 10 in the “prism” group and 10 in the “control” group. The sensory-motor effects of PA were evaluated by measurement of manual and visual straight-ahead, and also by precision of pointing without visual feedback before and after each PA session. The functional independence measure (FIM) was evaluated before and at 1, 3 and 6 months after PA, while SN severity was assessed using the Behavioural Inattention Test (BIT) before and 6 months after PA. Before the intervention, only manual straight-ahead pointing constituted a reproducible sensory-motor measurement. During prism exposure, a questionnaire showed that not a single patient were aware of the direct effects of optical deviation on pointing movement performance. The sensory-motor after-effects produced by the PA produced a more rapid reduction of the rightward manual straight-ahead, which was secondarily followed by visual straight-ahead. These sensory-motor effects helped to clarify the action mechanisms of PA on SN. At the conclusion of the 6-month follow-up, the two groups showed similar improvement, indicating that a weekly PA session over 4 weeks was not sufficient to produce long-term functional benefit. This improvement was correlated with the evolution of visual straight-ahead, which can be proposed as a marker for patients outcome.
1
Introduction
Spatial neglect (SN) has been defined as a singular difficulty to detect, respond to or orient one’s attention toward stimuli presented or represented on the contralateral side of a brain lesion, particularly in the right hemisphere . The syndrome aggravates the severity of the associated motor and sensory deficits and is a predictor of poor functional prognosis . Several rehabilitation methods have been proposed to reduce the behavioral bias on the side of the brain injury and the awareness deficit characterizing the contralateral hemi-space of SN; however the level of evidence has been poor (see review: ). Using a meta-analysis encompassing 23 randomized clinical trials (628 participants), Bowen et al. showed that most studies have assessed the repercussions of rehabilitation programs on the results of standardized tests; while only 15 of them evaluated the impact of these programs on daily activities immediately afterwards. Only 6 measured their impact at a later date. The currently available results show a significant effect favoring cognitive rehabilitation procedures, but this is only the case using the standardized tests evaluating SN. Accordingly, efficacy in daily activities has yet to be rigorously demonstrated.
Rehabilitation using prism adaptation (PA) is one of the most widely used methods, and also one of the most effective . Its effects involve a wide range of perceptual, cognitive and motor functions that are affected in SN (see reviews: ): visual neglect , somatosensory and haptic neglect, tactile extinction , auditory extinction , representational neglect , numerical representations and writing or wheelchair movement . Beneficial effects on postural imbalance have likewise been observed in patients following clinical SN remission .
The effects of PA have been shown to be surprisingly prolonged in time compared to known durations in healthy subjects. Since our initial study, in which we reported on effects lasting at least two hours after a few minutes of visuo-motor exercises using prisms , the prolonged effects after a single PA session on visual neglect and clinical manifestations including reading , writing and wheelchair driving have been shown to exist. More relevantly for rehabilitation, the effects were shown to be more durable after repeated sessions of adaptation. Several non-randomized and randomized studies have reported long-term effects (exceeding 5 weeks) following intensive rehabilitation involving two daily PA sessions over a 2-week period. In a controlled trial including 38 patients with SN (20 rehabilitated and 18 controls), only in the sub-group of patients with moderate SN was a functional benefit related to PA observed on hospital discharge .
To establish a rehabilitation protocol, it is necessary to measure the duration of the effects of a single PA session. The cognitive improvement reported after one session generally fails to exceed 24 hours following PA but can persist for several days, and at times as long as a week (review in: ). Given the decline of after-effects magnitude concomitant with the repetition of adaptation sessions, it was decided to space them out. Given the fact that the sensory-motor effects of PA tend to last several days , it was decided to test a regime consisting in one PA session a week. At the same time, we lengthened the rehabilitation period to one month, whereas in most previous therapeutic trials (e.g. ), it lasted two weeks. The main goal of this double-blind randomized controlled trial was to explore the effects of moderately “dosed” PA; we evaluated the effects of weekly PA sessions over 4 weeks on SN and daily life activities in chronic patients. The potential clinical interest of a less intense prism adaptation regime is that it may facilitate therapeutic management in ambulatory care.
The second objective of this study was to explore the development of spatial frames of reference during the recovery of neglect patients and to clarify the relationship between the sensory-motor after-effects of PA and the expansion of these effects in this cognitive sphere . In fact, dissociations between the two levels of action of PA have been reported , whereas in other studies a significant correlation between the consecutive proprioceptive effects and SN has been observed . The existence or non-existence of a quantitative relationship between the sensory-motor and the cognitive consequences of PA in cases of SN is of fundamental importance not only for the design and validation of pilot studies in healthy subjects, but also for establishing immediate and objective factors conducive to the therapeutic benefits of PA expected in a given patient. That is why this study includes measurement of the sensory-motor parameters through which it is possible both to monitor the development of the patients” spatial frames of reference and to quantify PA. These parameters are: manual straight-ahead (MSA), visual straight-ahead (VSA) and open-loop pointing without visual feedback, or open-loop pointing (OLP). Given the fact that some authors tend to confuse visuo-motor adaptation and error reduction during exposure to deviation (thereby leading to the erroneous conclusion that adaptation is deficient in neglect patients ), detailed exploration of the above parameters was directed at clarifying the means of measuring true prism adaptation . In addition, issues concerning alteration or facilitation of the adaptation process in neglect have been approached using a questionnaire evaluating patient awareness of prism deviation. The reliability and predictive value of these variables, which have yet to be described in the literature, have also been explored in this study.
2
Methods
2.1
Patients
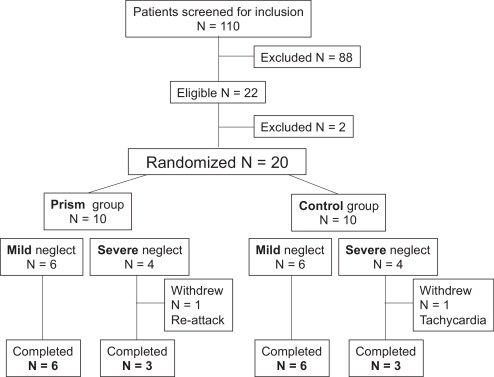
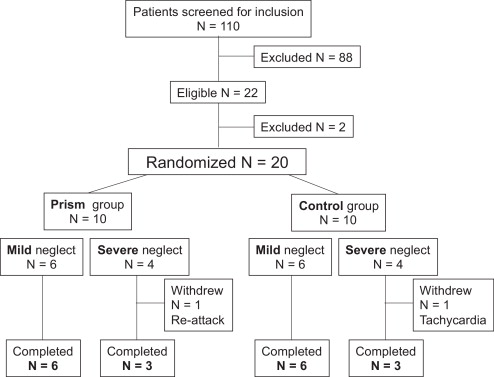
Nineteen patients admitted to the neurological rehabilitation medicine unit of Hôpital Henry-Gabrielle, Hospices Civils de Lyon and presenting with left SN secondary to a right-hemisphere stroke were included in the study from September 2001 until September 2005 ( Fig. 1 ). All patients were right-handed according to the criteria of the Edinburgh questionnaire .
The inclusion criteria were:
- •
age ranging from 18 to 90 years;
- •
a single stroke confirmed by a tomodensitometry examination or by brain MRI;
- •
left SN confirmed by several neuropsychological tests (line bisection test , balloon test , copy of a drawing, dictation and reading of a text);
- •
a time lapse of at least one month following the ischemic event.
The exclusion criteria were:
- •
existence of multiple brain lesions;
- •
temporo-spatial disorientation;
- •
psychiatric disorders;
- •
an associated, non-stabilized pathology.
The characteristics of the patients in the two groups are detailed in Table 1 .
| Case | N | Age/sex | LI | MD | SD | LHH | OCD | Delay | Aetiology | Lesion |
|---|---|---|---|---|---|---|---|---|---|---|
| Prism group | ||||||||||
| 1 | + | 40/F | 100 | 3 | 3 | P | 1 | 44 | Isch | (Frontal), parietal, occipital, temporal, insula, corona radiata, putamen |
| 7 | ++ | 40/F | 100 | 3 | 3 | P | 2 | 47 | Isch | Frontal, parietal, temporal, insula, corona radiata, putamen |
| 8 | + | 47/F | 100 | 3 | 0 | P | 1 | 34 | Isch | Frontal white matter, (corona radiata), insula, internal capsule, putamen, caudate nucleus |
| 9 | + | 69/M | 100 | 3 | 3 | P | 0 | 54 | Isch | Temporal, (occipital), corona radiata, internal capsule, putamen |
| 10 | ++ | 66/M | 16.66 | 3 | 3 | A | 2 | 60 | Isch | Frontal, parietal (temporal), corona radiata, putamen |
| 11 | + | 59/M | 100 | 2 | 2 | P | 1 | 88 | Hem | Parietal, occipital |
| 13 | ++ | 49/M | 100 | 3 | 3 | P | 1 | 30 | Isch | Frontal (temporal, parietal), putamen |
| 15 | + | 63/F | 100 | 3 | 3 | P | 1 | 42 | Isch | Corona radiata, capsule interne, putamen |
| 16 | ++ | 71/M | 100 | 3 | 3 | P | 1 | 60 | Isch | Frontal, parietal, corona radiata, putamen |
| Control group | ||||||||||
| 2 | + | 45/M | 83.33 | 3 | 3 | A | 1 | 35 | Isch | Frontal white matter, corona radiata, insula, internal capsule, putamen |
| 3 | ++ | 45/M | 100 | 3 | 3 | A | 1 | 92 | Isch | Frontal white matter, corona radiata, insula, putamen, caudate nucleus |
| 4 | ++ | 57/M | 100 | 3 | 3 | P | 1 | 38 | Isch | Frontal, temporal, parietal, insula, putamen |
| 5 | + | 72/M | 100 | 3 | 3 | A | 1 | 60 | Hem | Corona radiata, insula, internal capsule, putamen |
| 6 | + | 62/F | 91.66 | 3 | 3 | P | 1 | 46 | Isch | Parietal, occipital |
| 12 | + | 79/F | 100 | 2 | 2 | P | 1 | 38 | Hem | Parietal, occipital |
| 14 | ++ | 51/M | 100 | 3 | 3 | P | 2 | 67 | Isch | Temporal, parietal, occipital |
| 17 | + | 75/F | 100 | 1 | 1 | P | 1 | 34 | Isch | Occipital, parietal white matter, putamen, caudate nucleus |
| 18 | ++ | 69/F | 100 | 3 | 2 | P | 2 | 60 | Isch | Frontal, temporal, parietal, corona radiata, putamen, caudate nucleus |
2.2
Study description
This was a double-blind monocentric controlled randomized clinical trial involving 2 groups of patients presenting with left SN: a group undergoing PA rehabilitation, and a control group. The primary outcome measure of the study was the functional improvement in daily life activities following rehabilitation as assessed by the Functional Independence Measure (FIM) . This score was previously used in a non-randomized clinical trial evaluating the effectiveness of a trunk orthosis in SN rehabilitation in two groups of patients . The authors demonstrated, at 6 months, a statistically significant difference between the mean scores of the 2 groups (mean difference = 24; n = 11). In this study, the FIM end-point had a standard deviation of 10 units. Based on this variability measure, and to have at least a 90% chance of showing a 25-point difference between mean responses in the two treatments with a risk of type 1 error not exceeding 5%, 9 subjects had to be included in each group, (1–β = 0.90; β = 0.10; α = 0.05 and Δ = 25; n = σ 2 × M/Δ 2 = 100 × 54/625 = 8.6, i.e. at least 9 subjects).
Block randomization was carried out with the distinction of two levels of deficit severity used according to the initial seriousness of SN as assessed through the inclusion tests. Severe SN: was defined as deficit shown in all the inclusion tests, Schenkenberg score greater than 50° of deviation and BIT score ≤ 55. Moderate SN: was defined as deficit shown in some (from 1 to 4) of the inclusion tests, Schenkenberg score ranging from 11 to 50° of deviation and BIT score > 55.
The study was double-blinded: The examiners carrying out the evaluation (GR, SL, EM), did not know whether a given patient had undergone PA. They were distinct from the examiners performing the task of exposure to prismatic or neutral glasses (YR, SJC, LP). The double-blind procedure was facilitated by the fact that the SN patients were not aware of the disturbance induced by prism deviation and did not present the vegetative reactions expected during the appearance of motor errors when the prisms were worn for the first time (cf. infra and ). Consequently, they could be assigned without their knowledge to the “prism” and “control” groups. This also entailed that examiners performing the assessment did not receive information from the patients all of which that might have compromised the double-blind trial.
Block randomization and drawing by lots of the patients into the “prism” or “control” groups was carried out by Denis Pelisson, director of the ImpAct team in the Lyon Neuroscience Research Centre. The randomization was produced at 2 levels, firstly by selection of patients for the “prism’ or “control” group; secondly, by selection of patients according to the severity of the initial SN assessed by the BIT score, the objective being that the ratio of patients with severe neglect and patients with moderate neglect is comparable in the two groups.
All patients gave their consent to participate in the study. The experimental procedure was approved by the Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale Lyon B on 5 June 2001 le (Dossier 2001-040 B). The Hospices Civils de Lyon sponsored this study, which was registered on 2 August 2001 under the number 2001/0294. The study was financed by Inserm, the Hospices Civils de Lyon and the university Claude-Bernard Lyon 1.
2.3
Study parameters
The spatial frames of reference and precision of pointing at a visual target (without visual feedback) were measured from patient inclusion data through six months so as to monitor their evolution while the patients were recovering. Two measurements took place, before and after each exposure session, in view of quantifying the after-effects of prism adaptation.
The primary outcome measure for therapeutic efficacy was the functional score achieved according to the Functional Independence Measure (FIM) . The secondary outcome measure was the total score in the Behavioural Inattention Test (BIT) , which constitutes a good indicator of SN severity. Measurement with regard to the primary end-point was carried out 4 times: in pre-tests and in post-tests at 1 (M1), 3 (M3) and 6 (M6) months after the initial PA session. The BIT evaluations were performed twice: in pre-test, and then in post-test at 6 months. No intermediate evaluation took place during monitoring, in order to avoid the confounding effect of learning through repeated testing.
2.4
Prism adaptation and sensory-motor parameters
In the “prism” group, PA was carried out by wearing a pair of glasses producing a 10° rightward optical deviation of the visual field (OptiquePeter.com). The prismatic lenses were composed of two superimposed, curved, point-to-point lenses fitted with a “glacier” frame containing lateral leather protectors designed to avoid access to non-shifted vision. The prisms covered a total visual field of 105% in which each monocular field represented 75°, while the central visual binocular field represented 45°.
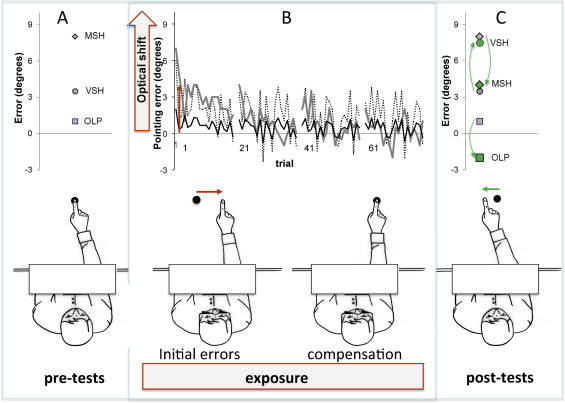
During prism exposure, the patient had to execute 80 rapid pointing movements towards visual targets located 10 degrees to the left or to the right of the middle of her/his body, the targets being made to pseudo-randomly alternate. In spite of repeated instructions to carry out rapid movements, the movements produced in brain-damaged patients generally remain too slow as to allow visual retroaction, and the errors committed by our patients did not necessarily reflect the amplitude of optical deviation or phase of adaptation ( Fig. 2 ). However, their degree of rapidity remained compatible with the development of actual sensory-motor adaptation by reducing the strategic components of compensation . The pointing movements were performed with a pause of 30 s after each series of 20, thereby favoring an increased number of errors at the start of the following series. During exposure, the patient did not see the initial position of her/his hand, which entered the visual field only once the movement was approximately 30 to 50% complete , in such a way as to favour proprioceptive-visual coding of the movement . All in all, prism exposure lasted from 6 to 10 mins (video tutorial: http://www.chu-lyon.fr/web/4531 ). While the “control” group patients carried out this visuo-motor task under the same conditions, they were wearing a pair of placebo glasses fitted out with neutral lenses of the same weight consisting in two 5° prismatic lenses set-up so as not to produce any optical deviation ; (OptiquePeter.com). Each patient carried out the exposure task (with prismatic glasses or neutral lenses) 4 times: at D0 (Expo1), at D + 7 (Expo2), at D + 14 (Expo3) and at D + 21 (Expo4). All of the exposure sessions took place under the same conditions and with the same operators.
The patients’ perceptual awareness of the optical deviation and its effects on movement trajectories were systematically studied using a phenomenological questionnaire (cf. Appendix A ). This open questionnaire consists in 20 questions divided into 3 main parts and progressing from open-ended formulations to highly specific questions on prism deviation. The first part includes 5 open questions put forward after ten preliminary pointing movements carried out prior to putting on glasses. The questions progress from (Q1: How is the exercise going?) and (Q3: Have you observed anything in particular?) to (Q5: Is it easy to aim toward the target?). The second and main part is administered after 5 movements carried out with glasses on, that is to say during the early exposure period, which usually generates maximal pointing errors . This consisted of 12 questions, the first of which are a reprise of the 5 preceding open questions (Q6 to Q10), while the following queries become increasingly explicit (Q11 to Q17) (Q17: In some patients these glasses can render it difficult to aim with the hand. How about you?). The third and final part of the questionnaire (Q18 to Q20) is given after 20 trials and at the end of active exposure to the prisms.
The after-effects of PA were evaluated by means of repeated MSA measurements in the dark ( n = 10), by VSA in the dark ( n = 10) and by OLP in the direction of a visual target ( n = 10). The VSA and MSA measurements present a double interest in the framework of our study as they are classically used independently to evaluate an egocentric reference and by subtraction of the measurements obtained before and after prism exposure (VS = visual shift and PS = proprioceptive shift) in view of quantifying the after-effects of PA . OLP is used to measure through the same subtraction operation (TS = total shift) the total after-effects of PA . The three sensory-motor parameters were evaluated in pre-test and in post-test at 1 (M1), 3 (M3) and 6 (M6) months in order to monitor the development of each patient’s frames of reference, and also before and after each prism exposure session (Expo1, Expo2, Expo3 and Expo4) in order to quantify the adaptation. To avoid any contamination of the sensory-motor parameters by cognitive parameters involved in compensation for prism deviation occurring independently of adaptation , we carefully ensured that the pre-test and post-test evaluations were not organized under the same conditions as prism exposure. The target used for OLP and the precision/rapidity instructions given differed from those employed during exposure.
MSA measurement was carried out by asking patients to point with their right hand in darkness in the “straight-ahead’ position in the direction of an imaginary line dividing their body into two equivalent halves. The subject was required to employ her/his arm without any speed or amplitude constraint, and if necessary, was reminded that she/he was not expected to proceed rapidly or stretch out her/his arm to the greatest possible extent. Pointing was measured using a contractor attached to a thimble threaded into the index finger, atop a table covered with isoresistant carbon paper on which two 65 × 1 cm electrodes were applied, thereby delimiting a section at an angle of 50° and a depth of 70 cm centered at the starting position near the torso. A direct 5 V current was generated between the electrodes. When the finger touched the surface of the table, tension between the thimble contact point and the reference electrode was measured as in a potentiometer. Tension measurement enabled us to calculate the angular position in relation to the objective sagittal axis, and this position could then be converted into degrees and conventionally signed (negative on the left, positive on the right) ( Fig. 3 A ). Measurement precision was estimated at ± 0.5 degrees.

VSA measurement was carried out in total darkness. The patient was comfortably seated in front of a table, with her/his head held straight up by a chinrest . A luminescent red diode was moved by the experimenter onto a 2-m horizontal ramp set face to the patient at a distance of 1 m. Speed of traverse ranged from 20 to 30 cm/s. Ten measures were successively carried out by alternating target movement in the left-right and right-left directions. The patient was asked to vocally interrupt (“Stop!”) the movement of the target as soon as she/he perceived it to be in a “straight-ahead” position ( Fig. 3 B). Measurement of the deviation was performed using a calibrated galvanometer and converted into angular deviation with regard to the objective straight-ahead position.
OLP accuracy measurement was carried out under the same conditions of darkness and with the same set of devices ( Fig. 3 C). The luminous visual target was aligned with the patient’s sagittal axis. The instruction given to the patients was to place their right hand at the target drip-line as precisely as possible but without time constraint, the goal being to distance themselves from the pointing conditions employed during exposure in view of obtaining measurements of sensory-motor after-effects that would be less influenced by cognitive factors .
During prism exposure, the terminal errors of each movement were captured by means of the thimble and converted into degrees of angular error with regard to the target.
2.5
Statistical analysis
First of all, the pre-test results of the two groups were compared. Initial variance analysis with repeated measurements were compared the two groups according to the parameters of age, FIM, BIT and mean time lapse after stroke. Sensory-motor parameters were compared by means of a repeated measures (RM) Anova including the “Session” factor since two pre-tests were available. Reliability of the sensory-motor measurements was evaluated using correlations.
Evaluation of the sensory-motor effects proceeded using two main steps. The first tests compared the different after-effects measured in terms of post-pre difference using unilateral Student t -tests against a theoretical value of zero. A three-factor Anova with repeated measurements then compared the two treatments (group factor, inter-subject: prism and control) with two intra-subject factors: pre-post (measured before and after exposure) and session (Expo 1 to 4). The error reduction curves produced during exposure could not be interpreted, due to highly variable and relatively slow movement speed.
In order to study the long-term impact of the 4 PA sessions on the sensory-motor variables, repeated measures analysis of variance was carried out with the “session” (pre, M1, M3, M6 or pre and M6), and “group” (prism, control) factors for each functional parameter under examination.
To conclude, the results enabled us to explore the predictive value of the sensory-motor parameters on the BIT and FIM scores, along with evolution between the pre-tests and M6.
2
Methods
2.1
Patients
Nineteen patients admitted to the neurological rehabilitation medicine unit of Hôpital Henry-Gabrielle, Hospices Civils de Lyon and presenting with left SN secondary to a right-hemisphere stroke were included in the study from September 2001 until September 2005 ( Fig. 1 ). All patients were right-handed according to the criteria of the Edinburgh questionnaire .
The inclusion criteria were:
- •
age ranging from 18 to 90 years;
- •
a single stroke confirmed by a tomodensitometry examination or by brain MRI;
- •
left SN confirmed by several neuropsychological tests (line bisection test , balloon test , copy of a drawing, dictation and reading of a text);
- •
a time lapse of at least one month following the ischemic event.
The exclusion criteria were:
- •
existence of multiple brain lesions;
- •
temporo-spatial disorientation;
- •
psychiatric disorders;
- •
an associated, non-stabilized pathology.
The characteristics of the patients in the two groups are detailed in Table 1 .
| Case | N | Age/sex | LI | MD | SD | LHH | OCD | Delay | Aetiology | Lesion |
|---|---|---|---|---|---|---|---|---|---|---|
| Prism group | ||||||||||
| 1 | + | 40/F | 100 | 3 | 3 | P | 1 | 44 | Isch | (Frontal), parietal, occipital, temporal, insula, corona radiata, putamen |
| 7 | ++ | 40/F | 100 | 3 | 3 | P | 2 | 47 | Isch | Frontal, parietal, temporal, insula, corona radiata, putamen |
| 8 | + | 47/F | 100 | 3 | 0 | P | 1 | 34 | Isch | Frontal white matter, (corona radiata), insula, internal capsule, putamen, caudate nucleus |
| 9 | + | 69/M | 100 | 3 | 3 | P | 0 | 54 | Isch | Temporal, (occipital), corona radiata, internal capsule, putamen |
| 10 | ++ | 66/M | 16.66 | 3 | 3 | A | 2 | 60 | Isch | Frontal, parietal (temporal), corona radiata, putamen |
| 11 | + | 59/M | 100 | 2 | 2 | P | 1 | 88 | Hem | Parietal, occipital |
| 13 | ++ | 49/M | 100 | 3 | 3 | P | 1 | 30 | Isch | Frontal (temporal, parietal), putamen |
| 15 | + | 63/F | 100 | 3 | 3 | P | 1 | 42 | Isch | Corona radiata, capsule interne, putamen |
| 16 | ++ | 71/M | 100 | 3 | 3 | P | 1 | 60 | Isch | Frontal, parietal, corona radiata, putamen |
| Control group | ||||||||||
| 2 | + | 45/M | 83.33 | 3 | 3 | A | 1 | 35 | Isch | Frontal white matter, corona radiata, insula, internal capsule, putamen |
| 3 | ++ | 45/M | 100 | 3 | 3 | A | 1 | 92 | Isch | Frontal white matter, corona radiata, insula, putamen, caudate nucleus |
| 4 | ++ | 57/M | 100 | 3 | 3 | P | 1 | 38 | Isch | Frontal, temporal, parietal, insula, putamen |
| 5 | + | 72/M | 100 | 3 | 3 | A | 1 | 60 | Hem | Corona radiata, insula, internal capsule, putamen |
| 6 | + | 62/F | 91.66 | 3 | 3 | P | 1 | 46 | Isch | Parietal, occipital |
| 12 | + | 79/F | 100 | 2 | 2 | P | 1 | 38 | Hem | Parietal, occipital |
| 14 | ++ | 51/M | 100 | 3 | 3 | P | 2 | 67 | Isch | Temporal, parietal, occipital |
| 17 | + | 75/F | 100 | 1 | 1 | P | 1 | 34 | Isch | Occipital, parietal white matter, putamen, caudate nucleus |
| 18 | ++ | 69/F | 100 | 3 | 2 | P | 2 | 60 | Isch | Frontal, temporal, parietal, corona radiata, putamen, caudate nucleus |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





