Laboratory Findings in Osteoarthritis
Roy D. Altman
As a clinical entity, osteoarthritis (OA) is a constellation of clinical, radiographic, and synovial fluid findings. To date there are no pathognomonic laboratory abnormalities. Blood and urine test results are usually normal, and synovial fluid analysis often yields abnormal but nonspecific results. Nevertheless, tests of body fluids (i.e., blood, urine, and synovial fluid) may serve to exclude other forms of arthritis and identify metabolic disorders that may be associated with secondary OA. This chapter addresses clinically applicable studies and some laboratory studies that may aid in the diagnosis of different subsets of OA.
Blood
Cellular Constituents
The cellular components of blood are quantitatively and morphologically normal in uncomplicated primary OA. The platelet count may rise slightly as an acute-phase reactant during certain flares but remains within the normal range.
Acute-Phase Reactants
Although the erythrocyte sedimentation rate (ESR) is most often normal, modest elevations in the ESR may be observed transiently during clinical exacerbations of OA. More persistent elevations may be related to generalized polyarticular OA.1 To a lesser extent, other acute-phase reactants2 may be elevated transiently. There is a nonspecific modest increase in ESR with increasing age. Because the prevalence of OA also increases with age, any modest increase in ESR in OA should be addressed with caution. Hence, marked ESR elevations (above 50 Gmm/hr) should alert the physician to an unrelated, coexistentdisease.
Studies have linked C-reactive protein (CRP) elevations but not ESR with clinical severity of OA of the hip and knee.3 CRP was significantly associated with disability (as measured by the Health Assessment Questionnaire scale), joint tenderness, pain, fatigue, global severity, and depression. Median CRP was 5.9 μg/mL. ESR was associated with only functional disability. CRP levels were higher in those with erosive (mean 4.7 mg/l), in contrast with nonerosive (mean 2.1 mg/l; P = 0.001), interphalangeal OA.4 Elevated CRP was associated with generalized versus nongeneralized OA.5 Increased CRP has been correlated with hip pain.6
In a longitudinal population-based study of knee OA, levels of CRP were higher in 105 women with radiographic OA compared with 740 women without OA. In the 4-year follow-up, median levels of CRP were higher in the 31 women whose disease progressed at least one Kellgren-Lawrence grade (median, 2.6 μg/mL) than in the ones whose disease did not progress (median, 1.3 μg/mL).7
No increase in CRP or relation to activity was noted in 274 patients with knee OA, where high levels of soluble receptors of tumor necrosis factor (TNF)-1 were associated with lower physical function, increased knee symptoms, and higher radiographic scores.8
In conclusion, it appears that there is a modest increase in ESR with age that is not related to OA. Sensitive techniques of determining CRP most often demonstrate a modest increase in OA. No studies of CRP elevation in OA have corrected for the presence of cardiovascular disease as another reason for an increase in CRP. The significance in the CRP for diagnostic purposes or in monitoring activity of OA is yet to be determined.
Serum Chemistry
Glucose
OA does not impair glucose tolerance. Conversely, however, diabetes mellitus may accelerate the OA process.9 In an epidemiologic survey of 1026 patients, the mean fasting plasma glucose level was significantly higher in patients with OA than in normal control subjects.10 Thus, screening tests for hyperglycemia are indicated in OA patients with early onset or inordinately severe joint disease. Hyperglycemia may suggest hemochromatosis or acromegaly.
Insulin-Like Growth Factor 1
Insulin-like growth factor 1 (IGF-1) serum concentration correlates with the presence and the growth of osteophytes in knee OA and the overall progression of the disease.11 Serum IGF-1 concentration was linked with the development of distal interphalangeal joint OA and more severe and bilateral knee OA in women.12
Insulin
Hyperinsulinemia may be a separate risk factor in the development or progression of OA.13 In a study of 48 overweight patients, those with OA of the knee had statistically higher serum insulin levels than did those without OA of the knee.
Calcium, Phosphorus, and Alkaline Phosphatase
Results of routine biochemical assessment of bone metabolism are normal in primary OA. Secondary OA from calcium pyrophosphate dihydrate (CPPD) crystal deposition disease (“pseudo-osteoarthritis,” McCarty types C and D14) (Table 11-1) may raise suspicion of underlying primary hyperparathyroidism.
Plasma growth hormone levels are normal in primary OA. However, in one study,15 radioimmunoassay growth hormone levels were elevated in menopausal women with OA compared with a control group. Elevation of serum phosphorus concentration in a patient with an OA-like arthropathy may suggest acromegaly.
A minor or marked increase in serum alkaline phosphatase (bone specific) and an increase in urinary levels of markers of type I and II collagen breakdown suggest Paget disease of bone.
Cholesterol
Serum cholesterol may be an independent risk factor for OA.16 Hypercholesterolemia and high serum cholesterol levels (3rd versus 1st tertile) were independently associated with generalized OA, mostly knee OA. There was no association between cholesterol levels and bilateral OA.
TABLE 11-1 LABORATORY ASSESSMENT OF DISORDERS ASSOCIATED WITH OR CAUSING AN OSTEOARTHRITIS-LIKE ARTHROPATHY | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Cartilage Matrix Components
Sensitive and specific assays for cartilage proteoglycan components and degradation products17 have been developed. Although of interest, serum sampling appears of limited value because of several metabolic factors, including dilution in serum from a single joint dysfunction and unknown influences of renal or liver function.18,19
Keratan sulfate in humans is a distinct and unique sugar derived primarily (95%) from articular and intervertebral disk cartilage. Elevations of serum keratan sulfate levels have been found in patients with OA, but there is a considerable variation in values in both cross-sectional and longitudinal studies.
Plasma levels of hyaluronate in OA were twice that in rheumatoid arthritis (RA) and seven times that in control subjects.20 Elevated hyaluronate was found to correlate with the patients’ functional capacity. Another study of 94 patients with tibiofemoral OA showed that serum hyaluronate values at entry correlated with disease duration, minimum joint space, and previous surgery.21 In this retrospective review, radiographic progression or knee surgery correlated with higher baseline hyaluronate levels.
Levels of serum hyaluronate failed to show a correlation with the Lequesne algofunctional index, duration of symptoms, CRP, or severity of radiographic changes. In a cross-sectional study, serum hyaluronan levels correlated with radiographic OA, ethnicity, sex, and age even after adjustment for multiple variables (P <0.0045).22 In a 2-year follow-up, those with knee OA and higher hyaluronan levels at baseline had faster radiographic progression (P <0.005).23 Serum hyaluronan was followed in a 3-year study of knee OA.23 Baseline hyaluronan levels did not correlate with progression. However, reduction in hyaluronan found at the first year correlated with radiographic progression at 3 years (r = 0.27, P = 0.02) (similar findings were present for an increase in serum osteocalcin).
Levels of serum hyaluronate failed to show a correlation with the Lequesne algofunctional index, duration of symptoms, CRP, or severity of radiographic changes. In a cross-sectional study, serum hyaluronan levels correlated with radiographic OA, ethnicity, sex, and age even after adjustment for multiple variables (P <0.0045).22 In a 2-year follow-up, those with knee OA and higher hyaluronan levels at baseline had faster radiographic progression (P <0.005).23 Serum hyaluronan was followed in a 3-year study of knee OA.23 Baseline hyaluronan levels did not correlate with progression. However, reduction in hyaluronan found at the first year correlated with radiographic progression at 3 years (r = 0.27, P = 0.02) (similar findings were present for an increase in serum osteocalcin).
In knee OA, elevation of cartilage oligomeric matrix protein (COMP) related to progression.24 COMP increases may directly relate to physical exercise, even walking.25,26 Pain of hip OA has been correlated with increased COMP.27 Of 81 patients observed for 5 years, progression was defined as those having a decrease of 2 mm or more in joint space on radiographic examination or those requiring knee surgery during the 5-year follow-up.28 Serum COMP levels increased by a mean of 6.42 μg/mL in patients whose OA progressed compared with a mean of 0.07 μg/mL in those whose OA did not progress. In another study of 48 with hip OA and observed prospectively with radiographs and serum samples, levels of COMP correlated with rapidly progressing OA of the hip.29 In the same study, serum levels of bone sialoprotein (BSP) correlated inversely with osteophyte grade and sclerosis grade in OA of the hip.
COMP related to progression of knee OA in a 5-year follow-up and patterns of progression suggested that knee OA progression is episodic or phasic.30 The authors suggested that large individual variation precludes the use of COMP for predicting progression, but that sequential COMP measurements may help identify OA progression.
Metalloproteinases
Stromelysin (matrix metalloproteinase 3 [MMP-3]) has been found to be elevated in the serum of patients with OA and correlated strongly with the articular index.31 Levels of collagenase (MMP-1) or of tissue inhibitor of matrix metalloproteinases (TIMP-1) were within normal limits. In a study of 36 patients, MMP-3 and MMP-9 were significantly increased in patients with rapidly destructive hip OA versus OA in patients awaiting total hip prosthesis.32
Sex Hormones
Serum sex hormones have been measured in patients with OA. An association has not been found between endogenous estrogen levels and OA or its severity.33
Miscellaneous Components
Nonspecific elevation of plasma substance P levels was seen in patients with OA compared with normal individuals, but it did not occur to the degree of elevation in patients with reactive arthritis.34
When there is a reduction of serum iron levels in patients with OA, it is secondary to another illness, such as gastrointestinal blood loss (increased iron-binding capacity) or “chronic disease” (reduced iron-binding capacity). Increased serum iron concentration in OA may be secondary to another illness (e.g., hemochromatosis).
Serum copper and ceruloplasmin levels are normal in patients with primary OA. Increased serum copper concentrations with secondary OA of the large joints may be the result of Wilson disease (Table 11-1). Wilson disease of small joints may be related to chondrocalcinosis.
Immunologic Studies
Studies of the immune system to date have failed to identify aberrant cellular or humoral immunity in the pathogenesis of OA.
Cellular Studies
Sensitization to proteoglycan antigens was demonstrated in 9 of 22 patients with OA by the lymphocytotoxin production test35 but in only 1 of 14 patients by the lymphocyte transformation test.36 It remains unclear whether this cellular immune response contributes to joint damage or reflects the incidental unmasking of proteoglycan antigenic sites during cartilage breakdown.
Humoral Studies
The prevalence of serum rheumatoid factor in OA parallels that observed in the general population. Because the frequency of rheumatoid factor increases with advancing age, low-titer serum rheumatoid factor is anticipated in 5% to 20% or more of patients with OA.37,38 Otherwise, circulating immune complexes have not been observed.39
Low-titer antinuclear antibodies may infrequently be encountered in patients with OA, with a prevalence equivalent to that of a similarly aged healthy population without OA.40,41
Antiproteoglycan antibodies have been noted in the serum of patients with severe OA.42 This finding appears to be an epiphenomenon of joint destruction and is not of etiologic or diagnostic significance.
Complement Studies
Levels of serum total hemolytic complement or specific complement components are normal. In one study,43 the ninth component of complement was elevated twofold in patients with OA compared with control subjects, similar to values observed in RA and nonrenal systemic lupus erythematosus.
Urine
Results of urine studies are normal in patients with primary OA. Urinary calcium and phosphorus levels vary widely, depending on dietary intake. Urinary estrogen and gonadotropin excretion is similar in postmenopausal women with and without OA.44
Urinary abnormalities may be noted in patients with some forms of secondary OA. In ochronosis, alkaline urine may darken on standing. Ochronotic urine reduces alkaline copper solutions, producing a false-positive result in the Benedict test for glycosuria. Confirmation requires enzymatic assay of urine (or serum) for homogentisic acid. Renal tubular acidosis associated with the OA of Wilson disease may produce hyposthenuria, glycosuria, aminoaciduria, proteinuria, and hyperuricosuria.
Any increase in urinary pyridinium collagen cross-links (pyridinoline and deoxypyridinoline) have failed to correlate with grades of severity of OA45 except in a study of women with OA of the knee.46 In one study, the elevation was more notable in patients with OA of the knee than in those with OA of the hip or hand. Treatment with intra-articular depocorticosteroids led to a decrease in levels of the urinary cross-links.47 In contrast, in another study, no elevation of urinary hydroxypyridinium cross-links was found in patients with OA compared with control subjects.48
Synovial Fluid
Synovial fluid in primary OA is generally considered “noninflammatory” (Ropes and Bauer classification type I49). However, increased volume of joint fluid, frequent decrease in viscosity, mild but significant pleocytosis, and modest elevation of synovial fluid protein indicate inflammatory synovitis (Table 11-2).
TABLE 11-2 SYNOVIAL FLUID FINDINGS IN NORMAL AND PRIMARY OSTEOARTHRITIC JOINTS | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
Volume
The volume of synovial fluid in the knee may vary from normal (0.5 to 1.5 mL)50 to greater than 100 mL in OA. In general, smaller increases in synovial fluid may be evident in other joints with OA. Indeed, the mechanisms that control the volume of synovial fluid are poorly understood and can be explained only partially by vascular hydrostatic pressure. Inexplicably, minimal radiographic evidence of OA may be associated with large synovial effusions; conversely, severe radiographic evidence of OA may elicit only minimal or no synovial effusion.
Intra-articular volumes of synovial fluid as determined by radiolabeling were high (109± 35 mL) and were higher than the volume that could be aspirated (59 ± 28 mL).50 Mean clearance rate for the synovial fluid was 0.039 ± 0.030 mL (SD) per minute, somewhat slower than fluids from patients with RA.
Appearance
The normal synovial fluid usually appears pale yellow, dark yellow, or clear. Along with an increased viscosity, it is reminiscent of the derivation of the term synovial (syn, like; ovum, egg “white”). It may infrequently be blood tinged or frankly bloody. Joint bleeding most often occurs in affected glenohumeral and unstable knee joints and is often associated with an acutely painful exacerbation, trivial trauma, or increased activity. Hemarthrosis may reflect “pinching” of synovium between contiguous osteophytes or irregular joint surfaces or, less frequently, a microfracture of subchondral or osteophytic bone or a tear of the rotator cuff (shoulder) or anterior cruciate ligament (knee). In these cases, the bloody fluid is evident throughout the arthrocentesis procedure, and the fluid often fails to clot. In contrast, bloody fluid resulting from traumatic aspiration technique clears during the course of withdrawal, or, alternatively, blood is seen to enter the syringe and mix with initially yellow fluid during joint aspiration. Repeated aspiration of bloody fluid from a single joint should suggest pigmented villonodular synovitis, particularly if the synovial effusion has a “port wine” color.
In primary OA, shed cartilage “shard” fragments are visible as floating white specks and particles. In OA associated with ochronosis, pigmented shards of cartilage may assume the appearance of ground pepper in joint fluid.51
Clarity
Synovial fluid in OA is clear and occasionally faintly turbid.
Viscosity
Synovial fluid viscosity is dependent on a protein-hyaluronic acid complex. The hyaluronate complex consists of an unbranched glycosaminoglycan macromolecule of approximately 2000 kDa composed in turn of polymerized disaccharide dimers of glucuronic acid-glucosamine coiled into a spherical or ellipsoid
conformation. This conformation allows the structure to occupy a solvent domain considerably larger than the volume of the polymer chain. Hyaluronate depolymerization or synovial membrane secretion of a poorly polymerized hyaluronate or a hyaluronate complex with altered conformation results in diminished viscosity.
conformation. This conformation allows the structure to occupy a solvent domain considerably larger than the volume of the polymer chain. Hyaluronate depolymerization or synovial membrane secretion of a poorly polymerized hyaluronate or a hyaluronate complex with altered conformation results in diminished viscosity.
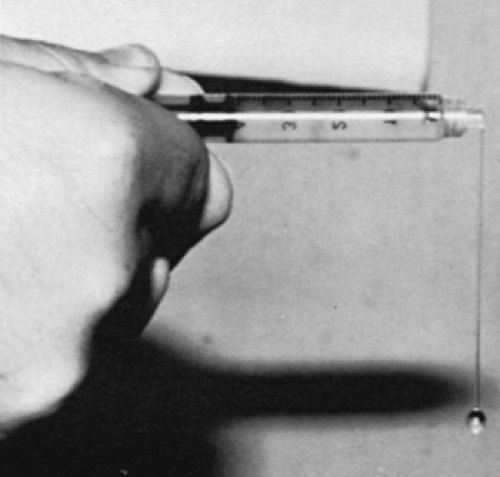
In OA, viscosity is inversely related to clinical evidence of inflammation; fluid from palpably “cool” joints usually has a normal viscosity and produces a “string” sign (Fig. 11-1). Markedly poor viscosity, in which the fluid drops like water from the syringe, is uncommon and may reflect coexistent pseudogout or another inflammatory arthritis. Conversely, extremely thick, viscous fluid should suggest osteochondromatosis or hypothyroidism.52 Pseudomucinous synovial cysts, found over the dorsum of osteoarthritic distal interphalangeal joints, contain pale, gelatinous fluid similar to that observed in ganglia.
Mucin
The precipitation of the protein salt of hyaluronic acid after acidification of joint fluid is the basis of the mucin clot or “Ropes” test.49 An aliquot of synovial fluid is added to a beaker containing a four times greater volume of 2% acetic acid and mixed with a glass rod. The resulting mucin clot (hyaluronate protein) reflects the degree of polymerization of hyaluronic acid. In OA, a tight, ropy mass is formed (graded good); whereas in RA and other inflammatory arthritides, the mass shows friable edges (graded poor). The mucin clot is almost invariably good in OA, even when viscosity is significantly diminished.
If it is uncertain whether synovial fluid has been aspirated, mucin clot formation and metachromatic staining are capable of detecting as little as 0.5 μL of synovial fluid.53
Synovial Fluid Microscopy
Leukocytes
Synovial fluid in OA may be relatively acellular, but a mild increase in the white blood cell count (1000 to3500 cells/mm3) often indicates inflammatory synovitis. Synovial pleocytosis in excess of 5000 cells/mm3 is uncommon (Table 11-2). The majority of leukocytes are lymphocytes (Table 11-2), predominantly T cells.54 Synovial fluid total and differential white blood cell counts can now be counted by an automated hematology analyzer by pretreatment with hyaluronidase at 37°C for 10 minutes.55
Cytoplasmic Inclusions
Leukocytes containing refractile intracytoplasmic inclusions by phase contrast microscopy are sparse in comparison with the numerous “ragocytes”56 of RA and other arthritides. In OA, these spherical inclusions, measuring 0.5 to 2.0 μm in diameter, appear to be composed largely of triglycerides57 (Fig. 11-2). In interphalangeal osteoarthritis, synovial cysts may contain large polymorphonuclear leukocytes containing multiple fat staining inclusions similar to those seen in ganglia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree