Abstract
Objective
To study the efficacy and safety of intrathecal baclofen therapy (ITB) in wheelchair-dependent adults with cerebral palsy.
Patients and methods
A retrospective analysis and clinical examination of 25 wheelchair-assisted adults with cerebral palsy receiving ITB initiated between 1999 and 2009 in three different cities in western France.
Results
ITB improves spasticity and facilitates wheelchair comfort and nursing care. The therapy has an effect on motor disorders and pain. Eighty percent of the ITB patients were satisfied. Dissatisfaction was related to complications or adverse events and not lack of efficacy. Complications occurred in 32% of the patients and transient interruption of the treatment or surgical removal of the ITB pump was necessary in 16% of cases.
Discussion and conclusion
Wider use of ITB in this indication is likely and should lead to a better understanding of the drug’s pharmacological effects on motor disorders and pain. Use of the Goal Attainment Assessment Scale or Caregiver Questionnaire can help us.
Résumé
Objectifs
Étudier l’efficacité et la tolérance du Baclofène intrathécal au long cours chez les patients adultes paralysés cérébraux non marchants.
Patients
Analyse rétrospective des dossiers et examen clinique de 25 patients paralysés cérébraux adultes non marchants implantés d’une pompe à Baclofène intrathécal et suivis à Angers, Le Mans ou Nantes entre 1999 et 2009.
Résultats
Le Baclofène intrathécal est efficace sur la spasticité et améliore par ce biais la station assise et le nursing. Il existe un effet du Baclofène intrathécal sur les mouvements anormaux et les douleurs. Quatre-vingt pour cent des patients porteurs de pompe à Baclofène intrathécal sont satisfaits et l’insatisfaction provient du fait de complications ou d’effets secondaires et non pas du manque d’efficacité. Les complications surviennent chez 32 % des patients nécessitant l’arrêt ou l’ablation de la pompe dans 16 % des cas.
Discussion et conclusion
Le développement du Baclofène intrathécal dans cette indication est probable et justifie une meilleure compréhension des effets pharmacologiques sur les mouvements anormaux et la douleur. L’utilisation du Goal Assessment Scale ou d’échelles de quantification de soins peuvent permettre de faciliter l’évaluation de l’efficacité du Baclofène intrathécal et son suivi personnalisé au long cours.
1
English version
1.1
Introduction
Cerebral palsy (CP) is caused by nonprogressive damage to the motor control centres of the developing brain. Regardless of the condition’s etiology, CP can occur during pregnancy, during childbirth or up until to the age of about three. Indeed, CP is an umbrella term encompassing a group of motor conditions which cause physical disability (mainly in the various areas of body movement and in the presence or absence of mental handicap) and problems in intrauterine development . CP is the leading cause of childhood disability in industrialized countries, with an incidence of 2 per 1000 live births. Despite progress in neonatal care, this value has remained stable over recent years. Life expectancy is barely over 30 in 87% of cases. The condition limits the patient’s activity and has a negative impact on quality of life .
The clinical symptoms of CP variously include motor impairments, sensory deficits (pain, loss of sight or hearing, etc.) and cognitive or intellectual impairments. It may also be associated with abnormal muscle tone (hypertonia or hypotonia), involuntary motion (with choreoathetosis or dyskinesia/dystonia in 7% of cases) and ataxia (in 5% of cases) . The Surveillance for cerebral palsy in Europe (SCPE) collaboration has proposed a simplified classification of CP, with five subgroups: unilateral spastic CP, bilateral spastic CP, dystonic CP, choreoathetotic CP and ataxic CP .
Spastic CP is by far the most common form of the condition and occurs in 80% of all cases with hypertonia (phasic or tonic patterns) . It triggers painful complications, orthopedic disorders (muscle retraction, joint deformity, scoliosis, etc.) and skin disorders (pressure ulcers) and has a negative impact on the patient’s comfort and quality of life .
Intrathecal baclofen therapy (ITB) is a unique treatment for treating spasticity in which the drug baclofen [4-amino-3-(4-chlorophenyl)-butanoic acid] is directly injected into the fluid surrounding the spinal cord in small, precise doses by an implanted pump. The technique has been used since 1984 and received marketing authorization in France in 1995. Its main indication is severe, generalized, functionally disabling spasticity which is refractory to conventional therapy . Compared with oral administration, the improved bioavailability of ITB limits the drug’s side effects on the central nervous system. On the pharmacological level, baclofen is a gamma-aminobutyric acid (GABA) agonist. It decreases the excitability of the spinal and brain stem motoneurons by regulating excitatory neurotransmitter release . Baclofen also decreases the muscle hypertonia caused by agonist/antagonist muscle co-contractions by modulating the spinal and cerebral monosynaptic and polysynaptic reflex activity of the afferent terminals, descending nerve fibers and regulating orthosympathetic system pathways .
ITB was approved for the treatment of spasticity in spinal cord pathologies (multiple sclerosis and traumatic spinal cord injury) in 1992 and in supraspinal pathologies (CP, brain damage and ischemic stroke) in 1996. Hoving et al.’s cost/efficacy study reported improved quality of life in these patients . In 1991, ITB started to be used to treat dystonia and help avoid irreversible rhizotomies . Intrathecal baclofen is one of the links in the global care chain for CP patients. In the management of spasticity and its consequences, ITB can be combined with physiotherapy, botulinum toxin A, neurotomy and orthopedic surgery.
Inevitably, ITB suffers from a number of technical complications (e.g. catheter failures or implanted pump incidents) and pharmacological complications (e.g. baclofen-related adverse events and dosage problems). This therapy requires rigorous, long-term medical monitoring of the patient by a specialist care team.
The first studies on ITB in CP were performed as case series in 1985 . Next, several prospective studies evaluated ITB’s action on spasticity , dystonia and movement execution . In children with CP, ITB was seen to have both an antispastic action and a positive effect on orthopedic complications . Subsequently, some authors reported a functional improvement, making nursing care easier and improving gait in children . Very few studies were performed on adult CP patients. ITB appears to be most effective in two particular stages of the Gross motor function measure (GMFM): patients walking with ortheses (GMFM II) and wheelchair-assisted patients with little or no mobility (GMFM V) . Whereas other researchers have performed in-depth studies of walking CP patients (GMFM I, II and III) , we chose to evaluate the subgroup of wheelchair-assisted adults (GMFM IV and V) in terms of spasticity, activity limitation, treatment satisfaction and quality of life. To the best of our knowledge, this topic has not been reported in the literature .
The primary objective of this retrospective study was to describe the long-term efficacy of ITB in 25 wheelchair-assisted adults with CP in terms of impairments, activity limitation, participation restriction and quality of life. The secondary objectives were to assess drug safety (by studying the complications) and to evaluate satisfaction with ITB (as expressed by the patients, family and caregivers).
1.2
Patients and method
1.2.1
Population
This retrospective study covered a cohort of 25 wheelchair-assisted adult CP patients with intrathecal baclofen pumps implanted between 1999 and 2009 in three cities in western French (Angers, Le Mans and Nantes). We recruited 14 men and 11 women (mean ± standard deviation [S.D.], age: 29.6 ± 12.66). The inclusion criteria were as follows: a diagnosis of CP, complete wheelchair dependence and age greater than 15 at the time of pump implantation. All patients and/or their families received detailed information on the study and gave their written, informed consent to participation.
According to the SCPE classification, the cohort included 84% bilateral spastic CP patients and 16% choreoathetotic CP patients ( Table 1 ). On the functional level, 76% of the patients were dependent for maneuvering their wheelchair and 28% were able to answer the questionnaire unaided.
| Clinical types | |
| Unilateral spastic CP | 0 |
| Bilateral spastic CP | 21 |
| Choreo-athetotic CP | 4 |
| Dystonic CP | 0 |
| Ataxic CP | 0 |
| Lesion level | |
| Quadriplegia | 21 |
| Diplegia | 4 |
| Wheelchair dependence | |
| Third party dependent | 19 |
| Independent | 6 |
1.2.2
The implantation protocol
The indication for implantation of an ITB pump was functional impairment caused by treatment-refractory, generalized spasticity and a modified Ashworth score greater or equal to 3 . A clinical test was performed to validate the indication for pump implantation, in accordance with the objectives defined with the patient and his/her family but also in light of the reported adverse events. The therapeutic goal was to decrease the Ashworth score by at least one point. The main functional goals were to facilitate nursing care (washing and getting dressed), improve wheelchair comfort, reduce dystonic or choreoathetotic movements, improve mobility (wheelchair transfers and maneuvering) and relieve spasticity-induced pain. The test was performed via either a spinal tap and baclofen bolus infusion of 25 to 100 μg or by implanted catheter and bolus every 24 to 48 hours or continuous baclofen administration. An evaluation of the risk/benefit ratio took into account the clinical safety, the dose level used, effects on spasticity six hours after the injection (on the Ashworth scale) and the predefined functional objectives. If the patient, his/her family, the care team and other involved healthcare professionals were satisfied with the efficacy of the ITB tests, pump implantation was then scheduled.
After pump implantation, we collected the data on dose level changes at 1, 3, 6 and 9 months post-surgery and then every year after that.
Post-surgery adverse events involving the baclofen pump were classified as “complications” if they required renewed hospitalization and “adverse events” if symptomatic treatment alone was administered on an outpatient basis. The incident rate was reported in pump-years, corresponding to the number of years of ITB.
1.2.3
Evaluation
Our retrospective analysis of the patient’s medical records examined the patients’ personal medical history (epileptic seizures, neuro-orthopedic surgery, pressure ulcers, etc.) and their clinical symptoms (pain and pressure ulcers) and preoperative evaluations (an orthopedic check-up, the spasticity level on the modified Ashworth scale, spinal status, etc.). All patients were seen again between June 2008 and June 2009 for the observational evaluation of spasticity on the Ashworth scale and the assessment of the long-term consequences of ITB therapy. The patient’s functional status was evaluated according to the degree of autonomous mobility (i.e. dependency on a third party or independent wheelchair use) and cognitive function was evaluated according to the patient’s ability or inability to answer the questionnaire.
The efficacy of ITB was subjectively assessed in a questionnaire ( Annex I ) filled out by the patient or his/her family (depending on the patient’s cognitive disorders) some time after the implantation of the baclofen pump. We measured satisfaction on a visual analog scale (VAS), since it is known to be very easy to use for patients with cognitive disorders. The various areas studied were as follows: the change over time in impairments (pain, spasticity and movement control), activity limitations (nursing, physiotherapy care, transfers, wheelchair mobility, meals, bladder/bowel disorders and sleep), quality of life and overall satisfaction with ITB. The pretherapeutic objectives were achieved when the patients reported an improvement of more than 5 out of 10 on the VAS. The evaluation was completed by an item asking explicitly whether or not the patient would again choose to have an ITB pump implanted. Space was also available for additional comments.
In view of the small sample size, our use of retrospective data and the absence of a control group, the statistics computed are reported as the mean, S.D. and range for quantitative variables or as a percentage for the qualitative variables.
1.3
Results
1.3.1
Technical information
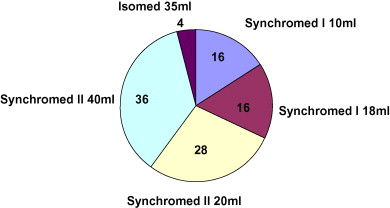
All implanted pumps were programmable models (24), except for one with continuous flow. Most were Medtronic SynchroMed ® II devices (16), with a predominance of 40 ml pumps (nine) ( Fig. 1 ). The intrathecal catheter was implanted at the mid-thorax level in eight cases (32%), the upper thorax level in five cases (20%) and the thoracolumbar level in four cases (20%). Position data was not available in seven cases.
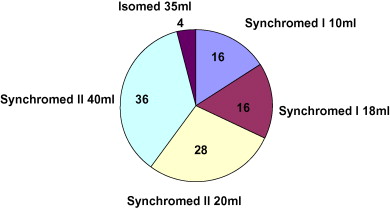
All but one of the patients received a 2000 μg/ml baclofen solution. The individual daily baclofen dose over the long-term varied from 25 μg to 1015 μg per day, with a mean value of 292 ± 106.4 μg per day ( Fig. 1 ). During the first year, the daily baclofen dose was often below than 100 μg per day, with a mean value of 128 ± 97 μg per day baclofen (range: 40–500). The dosage increased by 103% over the first year and then more or less stabilized, with a 9.8% yearly increase. At 5 years post-implantation, the doses ranged from 96 μg to 1015 μg per day; most doses were below 400 μg per day (seven out of 11), with a few higher doses at 750 μg per day (four out of 11) ( Fig. 2 ).
The total number of years of follow-up was 116 (for 25 patients). In two patients, the pump battery had to be replaced after 6 and 8 years, respectively.
1.3.2
Efficacy
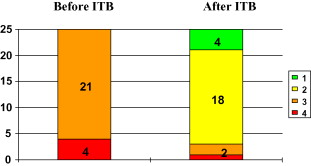
The clinical efficacy of ITB was confirmed by the change over time in the modified Ashworth scale [from an average of 3.2 ± 0.4 (range: 3–4) before initiation of therapy to 2 ± 0.6 (range: 1–4) afterwards ( Fig. 3 )].
As mentioned above, the limited degree of cooperation by certain patients with cognitive disorders prompted us to measure the functional efficacy of ITB was analyzed using a simple, subjective satisfaction scale (VAS), ( Annex I ). In terms of the main objectives set prior to pump implantation, 96% of the patients were seeking facilitated nursing (washing, getting dressed) and the mean satisfaction score was 6 ± 3.3 (range: 0–10); 88% of the patients were seeking improved wheelchair comfort and the mean score was 6 ± 3 (range: 0–9). Twenty-four percent of the patients ( n = 6 out of 25) wanted a decrease in abnormal choreoathetotic movement and the mean satisfaction score was 3 ± 4.1 (range: 0–8). The objectives were not at all reached (VAS score = 0 out of 10) for three patients and clearly reached (VAS score = 7 or 8 out of 10) for two patients. One patient passed away during the follow-up period (due to pneumonia) and could not be evaluated. For these six patients, one of the two catheters placed in an upper thorax position operated successfully and only one of the four catheters implanted in a thoracolumbar position was successful.
In all, 80% of the patients ( n = 19) considered that their main ITB objectives has been achieved (VAS score greater than 5 out of 10).
Furthermore, 88% of the patients (22) experienced improvements other than those wished for in the treatment objectives; these included pain relief in 68% of cases ( n = 15 out of 22), easier movement execution in 45% of cases (10 out of 22) and better sleep in 23% of cases ( n = 5 out of 22).
More generally, 72% reported a better quality of life (VAS score greater than 5 out of 10), regardless of whether their main objectives were achieved or not. The overall ITB satisfaction score was reported 7 ± 3.2, with 80% of satisfied patients (VAS satisfaction score greater than 5 out of 10). In response to the question “Would you go through intrathecal baclofen pump treatment again?”, 68% answered “yes” ( n = 17 out of 25), five answered “no” and three did not reply. Of the five patients who would have wished to go through the procedure again, none cited lack of ITB efficacy as a reason. In fact, two patients reported complications requiring several hospital stays and three reported that the discomfort experience as a result of adverse events outweighed the expected benefits.
1.3.3
Complications and adverse events
Following pump implantation and with a total of 112 pump-years, 72% of the patients presented complications or adverse events ( n = 21 out of 25). Eight incidents were classified as complications (0.07 per pump-year, Table 2 ) requiring hospitalization, including four cases in which the ITB had to be interrupted or the pump had to be surgically removal. These were one case of iatrogenic section of the catheter, one case of pump infection (both were secondary to vertebral fusion, i.e. arthrodesis) and two requests for stopping the treatment after the patients suffered from psychiatric decompensation. One case of severe, chronic constipation is still being discussed, in order to evaluate the ITB risk/benefit ratio in this patient. Thirteen incidents were adverse events which resolved themselves spontaneously or after symptomatic treatment, with no after-effects.
| Complications | NBE | Observation | Treatment | End ITB? | |
|---|---|---|---|---|---|
| Technical | Catheter breakage | 1 | Idiopathic catheter breakage Catheter breakage during vertebral fusion (arthrodesis) | Surgical treatment: Changing the catheter Under discussion | No Yes |
| Medical | Infection | 1 | Postoperative (MRSA): inflammatory pump + purulent discharges | Surgical treatment: washing + combination antibiotic therapy | No |
| Infection during vertebral arthrodesis | Surgical treatment: Pump removal + arthrodesis removal | Yes | |||
| Constipation | 1 | Constipation with bowel sub-occlusion | Under discussion | ? | |
| DVT | Left femoral DVT | Medical treatment | No | ||
| Psychiatric | Anxious-depressive Syndrome | 1 | Pain increase and multiple psychiatric symptoms as adverse events | Pump stopped | Yes |
| Anxiety before filling the pump and dysmorphophobia | Surgical treatment: patient asked for surgical removal of the pump | Yes | |||
Hence, 28% (7 out of 25) of the patients with ITB pumps had no complications or adverse events ( Table 3 ). Two of these patients had a known epileptic condition prior to surgery and the latter was not impacted by the ITB.
| Adverse events | NBE | Observation | Treatment | |
|---|---|---|---|---|
| Postoperative | Spinal tap syndrome | 3 | Headaches, nausea, vomiting | Medical treatment |
| CSF effusion | 2 | Subcutaneous collection around the pump and non-inflammatory | ||
| Pressure ulcer | 1 | Pressure ulcer at the location of the implanted pump | ||
| Pharmacological | Bladder/Bowel | 2 | Aggravated urinary incontinence | Medical treatment |
| 1 | Dysuria (painful urination) | Monitoring | ||
| Swallowing disorder | 2 | Excessive salivation and more frequent choking on food | Preventing swallowing disorders + treatment for aspiration pneumonia | |
| Pruritus | 1 | Pruritis since the implantation and after each filling | Treatment with an H1 antihistamine | |
| Edema of the legs | 1 | Edema of the legs with high doses | Decreased ITB doses + support tights | |
1.4
Discussion
1.4.1
The study population
Our cohort of 25 wheelchair-assisted CP patients from the Loire region of western France is demographically representative of CP patients, with a male/female ratio of 1.27 (versus 1.3 in the literature) . Our study focused exclusively on wheelchair-assisted adult patients, who represent about 25% of adult individuals with CP . In 2008, this corresponds to about 20,000 individuals in France, if we assume that the prevalence of CP is 1.25 adults per 1000 inhabitants . The actual estimation in our studied region would be 1000 wheelchair-assisted adults with CP, given that the Loire region represents 5% of the population of mainland France and Corsica (according to data from the French National Institute of Statistics and Economic Studies published in 2009). Even though, spasticity is a common impairment in CP (85% of cases), only 2.5% of wheelchair-assisted patients are actually treated . The infrequent use of ITB in this population may correspond to a misunderstanding of this therapy, indifference towards this dependent population (requiring third party assistance for mobility in 76% of cases) or apprehension of the specific risks (which are probably greater than in other populations).
The evaluation of wheelchair-assisted patients with CP is complicated by the presence of associated communication disorders and intellectual impairments in 66% of cases and by the fact that no consensus has been established concerning the assessment procedure to be used in this population. In the present study, subjective assessment of ITB’s efficacy was performed with an easy-to-use VAS for satisfaction. This enabled us to establish the patient’s opinion early on by using a self-assessment scale or, for more severely handicapped patients, an observational evaluation with family members or caregivers .
Data were missing for one patient, who died from pneumonia. This death was not correlated to implantation or use of the ITB pump. In fact, lung infections are a common cause of death in patients with CP, especially when there is intellectual impairment and mobility limitation .
1.4.2
Efficacy in various indications
The long-term efficacy ITB for spastic hypertonia was confirmed here by a 1.2-point improvement on the modified Ashworth scale (3.2 before therapy and 2 afterwards) . This improvement appears to be smaller than in other spine-related spastic pathologies. However, the average doses used here were rather low (292 ± 106.4 μg per day [range: 25–1015]) relative to the literature values (470 μg per day in average with a maximum of 1700 μg per day) , especially for quadriplegics (84% of cases) ( Table 1 ) which are associated by higher baclofen needs . Furthermore, given that no overdose-related adverse events (such as hypotonia, sleepiness and respiratory distress) were reported, we believe that it would have been possible to increase the dose and improve the efficacy of ITB. The pharmacology of ITB in CP has been partially characterized; it has the same clinical efficacy as in spinal cord pathologies but requires higher doses . An increase in the efficacy of ITB following dilution of the drug has been reported . We did not observe this phenomenon in our population; only one patient had a concentration of 1000 μg/ml and most other patients had a concentration at 2000 μg/ml.
Only two patients stated that the reduction in abnormal choreoathetotic movements was satisfactory. This could be explained by the fact that no catheters were implanted at the upper thorax level in these quadriplegic patients, although Rawlins and Albright suggested placing the catheter at the cervicothoracic level . Albright and Ferson even go as far as to suggest placing the catheter’s tip in the third ventricle for intraventricular infusion and improvement of baclofen therapy’s efficacy on dystonia . However, choreoathetosis and dystonia are among the predominant abnormal movements in CP patients . Baclofen’s action on dystonia is poorly known but it seems to have an impact on the brain by reinforcing the inhibition of the putamen and corpus striatum on the pallidum via a decrease in excessive stimulation of the additional motor area . Abnormal movements are a common cause of disability and very few drugs give completely satisfactory results. Bilateral deep brain stimulation (DBS) of the central gray nuclei (i.e. the pallidal or thalamic nuclei) gives very encouraging results for patients with dystonic/choreoathetotic CP . Some authors have reported a certain degree of efficacy for ITB on opisthotonos in CP patients . It is possible that a combination of a better understanding of ITB’s mechanisms of action and changes in surgical techniques for implanting the catheter at a higher level could enable further comparative studies of DBS and ITB . Therapeutic indications for surgery are always based on a decision multidisciplinary care team, in total agreement with the wishes of the patient and his/her family.
ITB improved other impairments, such as pain (in 68% of cases) and sleep (in 23% of cases). This could be explained by an antispastic action on hypertonia, painful nighttime spasms and the ITB-specific analgesic effect demonstrated by Becker et al. . Given the prevalence of pain in 78% of individuals with CP, the treatment’s efficacy should be assessed prospectively . Some studies have focused on ITB’s efficacy on other impairments sometimes associated with CP, such as neurovegetative disorders in individuals with severe brain injury , communication, feeding disorders and alertness and awareness in patients in a persistent vegetative state .
On a functional level, the efficacy of ITB was satisfactory, since 80% of the patients felt that their main objective was reached (with a mean VAS satisfaction score of 7 ± 3.2). The most improved indications are predominantly in the area of comfort, such as wheelchair use and nursing care in wheelchair-assisted patients with CP . In the present study, ITB improves the quality of life in 72% of cases (regardless of whether their main objectives were reached or not), a figure which is in line with the literature data .
Most of our patients with an ITB pump were satisfied with it (80%). All of the five patients (20%) who answered “no” to the question “would you do it again?” attributed their reply to the occurrence of adverse events, rather than ITB’s lack of efficacy .
1.4.3
Complications and adverse events
Thirty-two percent of the patients presented complications (i.e. requiring a hospital stay), with an overall rate of 0.07 complications/pump-year (eight complications for 112 pump-years). This rate is comparable to the literature data . Some patients required surgical interventions for multiple catheter changes and pump infection ( Table 2 ); other had repeated hospitalizations for aspiration pneumonia secondary to swallowing disorders or bowel sub-occlusion episodes. Stopping and/or surgically removing the pump were required in two cases after vertebral fusion (infections and catheter breakage) and following two requests related to psychiatric decompensation (nonsystematic multiple pain and phobia of filling the pump).
Some adverse events appeared to be more CP-specific, such as psychological decompensation, swallowing disorders, constipation and technical problems associated with the aftermath of vertebral fusion (arthrodesis). In CP, scoliosis and spasticity are often associated and so the combination of vertebral arthrodesis and ITB is frequent in this population . We observed two complications involving vertebral fusion and ITB: an infection and iatrogenic catheter breakage. However, a recent retrospective study with four arms (A: 26 vertebral fusions before ITB; B: 11 simultaneous vertebral fusions and ITB; C: 25 vertebral fusions after ITB; D: 103 ITB alone) reported similar rates of infectious and catheter complications in all groups . The effect of ITB on scoliosis progression is still subject to debate. Although we did not specifically address this issue in the present study, none of the patients presented a major change over time in scoliosis after the implementation of ITB treatment .
In patients with CP, choice of the pump volume is important because sometimes very malnourished individuals run a risk of developing pressure ulcers on the pump implantation area . Furthermore, some patients are psychologically weak and present psychiatric decompensation associated with dysmorphophobia towards the operated area and pump volume . In fact, CP can be associated to personality and body representation disorders, which must be screened for prior to implantation .
Technical complications were rare (relative to older series ) because there was only one case of catheter migration and no pump problems in our study. Technical and surgical advances will probably decrease the incidence of pump and catheter complications in the future.
1.4.4
Evaluation of intrathecal baclofen therapy
In CP, evaluating the efficacy of ITB is complicated by the frequent presence of intellectual deficits, the heterogeneous nature of the clinical cases, the patients’ age range (adults and adolescents) and low degree of independence. We used standardized evaluation scales (such as the Ashworth scale for spasticity) because it is easy to use objective scale in clinical practice. For pain evaluation, observational measurement scales are more complex, due to the multiple nature of pain complaints when cognitive disorders are also present. Several French language scales for pain complaints in adults or adolescents with multiple disabilities (such as the expression de la douleur adulte ou adolescent polyhandicapé [EDAAP]) are currently being validated. The EDAAP is based on the Doloplus scale used in adult patients with cognitive disorders and the San Salvadour observational pain scale in children with multiple disabilities .
These scales measure spasticity as an impairment but do not evaluate its consequences in terms of patient comfort. To the best of our knowledge, no functional or quality of life scales have been specifically validated for patients with CP . Furthermore, generic scales (such as the Functional independence measure) do not show high sensitivity to change in this population . Questionnaires designed to measure the care given to the patients (such as the Caregiver Questionnaire) have also been designed. Other authors have transformed the latter into the Care and comfort hypertonicity questionnaire .
The objectives of ITB therapy in CP appear to depend on the nature of the patient’s impairments and level of personal independence. Scales such as the Goal attainment scale can evaluate personalized goals established prior to treatment initiation by the physician, the patient and his/her family. Some authors recommend the Goal Attainment Scale as a means of therapeutic evaluating antispasticity treatments , when used by trained, specialist teams , this evaluation is reproducible, has good sensitivity change and could enable long-term patient monitoring after the implantation of an ITB pump .
1.5
Conclusion
ITB is moderately effective as an antispasticity treatment in individuals with CP. Even though the efficacy is not as good as that reported for spinal cord pathologies, this treatment can improve wheelchair comfort and nursing care of wheelchair-assisted CP patients suffering from spasticity which is refractory to conventional treatments. This therapy requires surgery and regular follow-up of the patient and can only be envisioned after a multidisciplinary care team decision with the informed consent of the patient and his/her family. At present, no other treatments have been validated for the indication of refractory spasticity.
Relative to the potential target population, use of ITB in this indication appears to be limited by its moderate efficacy, specific complications in multiple disabilities, poor awareness among physicians and lack of referral to specialist consultations.
ITB was seen to have positive impacts on pain and movement execution. Advances in understanding both the pathophysiology of the disorders and ITB’s pharmacology could help better define the therapy’s indications. Technical progress in terms of the catheter implantation location is also very likely. It will also be necessary to compare ITB with other recently introduced therapies, such as DBS .
Conflicts of interest statement
The authors have not declared any conflicts of interest.
2
Version française
2.1
Introduction
La paralysie cérébrale résulte de lésions cérébrales pré-, péri- ou postnatales stables du système nerveux central en développement quelle que soit leur étiologie. Elle regroupe les notions d’infirmité motrice d’origine cérébrale avec ou sans déficience intellectuelle et les affections prénatales malformatives ou infectieuses . Elle représente la première cause d’incapacité chez l’enfant dans la plupart des pays développés avec une incidence de 2/1000 naissances. Malgré les progrès de la néonatalogie, ce taux reste stable et l’espérance de vie de ces adultes en devenir est supérieure à 30 ans dans 87 % des cas avec des répercussions en termes de limitation d’activité et de qualité de la vie .
L’expression clinique de la paralysie cérébrale est polymorphe, elle associe des déficiences motrices, sensorielles (douleurs, déficit visuel ou auditif), cognitives ou intellectuelles. Elle peut associer un trouble du tonus (une hypertonie ou une hypotonie), des mouvements anormaux (chorée-athétose ou dyskinésie dystonie dans 7 % des cas) et une ataxie dans 5 % des cas . La Société européenne de la paralysie cérébrale (SCPE) a proposé une classification simplifiée en cinq sous-groupes : les formes spastiques bilatérales, spastiques unilatérales, dystoniques, choréo-athétosique et les formes ataxiques .
La spasticité est fréquente dans la paralysie cérébrale avec 80 % de formes hypertoniques (phasiques ou toniques) . Elle génère des complications douloureuses, orthopédiques (rétractions musculaires, déformations articulaires, scoliose) et cutanées (escarres), source d’inconfort et de diminution de la qualité de vie .
Le Baclofène intrathécal (BIT) est un traitement antispastique locorégional utilisé depuis 1984 et dont l’Autorisation de mise sur le marché (AMM) date de 1995. Son indication principale est la spasticité sévère et diffuse, gênante fonctionnellement, résistante aux thérapeutiques classiques . Sa meilleur biodisponibilité par voie intrathécale limite les effets secondaires centraux par rapport à la voie per os. Sur le plan pharmacologique, l’acide 4-amino-3 (4-chlorophényl) butanoïque est un agoniste de l’acide gamma aminobutyrique (GABA). Il diminue l’excitabilité des motoneurones intramédullaires et intracérébraux en régulant l’afflux de neurotransmetteurs excitateurs . Il réduit l’hypertonie musculaire due aux co-contractions musculaires agoniste/antagoniste en modulant l’activité réflexe mono- et polysynaptique cérébrale et médullaire des terminaisons des fibres afférentes, des fibres descendantes et des voies de régulation du système orthosympathique .
Le BIT obtient l’agrément en tant qu’antispastique en 1992 dans les pathologies spinales (sclérose en plaques et blessé médullaire) et en 1996 dans les pathologies supraspinales (paralysie cérébrale, traumatisme crânien, accident vasculaire cérébral). Hoving et al. ont étudié son rapport coût-efficacité, celui-ci est satisfaisant compte tenu de l’amélioration de la qualité de vie des patients . Il a ensuite été utilisé dans les dystonies dès 1991 . Il permet d’éviter les radicotomies ou les Dreztomies irréversibles . Le BIT est un des maillons de la prise en charge globale du patient paralysé cérébral. Il s’associe à la kinésithérapie, l’appareillage, la toxine botulique, les neurotomies et la chirurgie orthopédique dans la maîtrise de la spasticité et de ses conséquences.
Les complications existent, elles peuvent être techniques avec les incidents de cathéter ou de pompe ou pharmacologique dues aux effets indésirables du Baclofène (sur dosage et sevrage). Cette thérapeutique nécessite donc un suivi médical rigoureux au long cours par des équipes spécialisées.
Les premières études du BIT dans la paralysie cérébrale furent effectuées sur des séries de cas en 1985 . Puis, des études prospectives évaluèrent son action sur la spasticité , la dystonie et l’amélioration de l’exécution des mouvements . Chez les enfants paralysés cérébraux, le BIT a démontré son effet antispastique et son efficacité sur la prévention les complications orthopédiques . Certains auteurs ont ensuite constaté l’amélioration fonctionnelle telle que la facilitation des soins et l’amélioration de la marche chez les enfants . Chez les adultes paralysés cérébraux, peu d’études ont été réalisées. L’efficacité du BIT serait meilleure pour deux stades fonctionnels de la gross motor function measure (GMFM) : les patients déambulants avec orthèses (GMFM II) et de ceux non déambulants avec peu ou pas de mobilité (GMFM V) Alors que d’autres équipes ont étudié plus particulièrement le sous-groupe de patients déambulant (GMFM I, II et III) . Nous avons choisi d’évaluer le sous-groupe de patients adultes non déambulants (GMFM IV et V) à la fois sur le plan de la spasticité, des limitations d’activités, de la satisfaction du traitement et de l’amélioration de la qualité de vie, car cela n’avait pas été réalisé à notre connaissance .
L’objectif principal de ce travail rétrospectif est d’étudier l’efficacité du BIT au long cours chez 25 patients paralysés cérébraux non marchants implantés en précisant les résultats sur les déficiences, les limitations d’activité, les restrictions de participation et la qualité de la vie des patients. Les objectifs secondaires sont, d’une part, d’évaluer la tolérance thérapeutique par l’examen des complications et, d’autre part, d’étudier la satisfaction des patients, familles ou soignants du BIT.
2.2
Patients et méthode
2.2.1
Population
L’étude rétrospective des implantations de pompe à Baclofène entre 1999 et 2009 à Angers, Le Mans et Nantes a recensé 25 patients paralysés cérébraux adultes non marchants. On recense 14 hommes et 11 femmes, d’âge moyen de 29,6 ± 12,66 ans. Les critères d’inclusion furent la présence d’une paralysie cérébrale, l’absence de déambulation possible et un âge d’implantation supérieur à 15 ans. Tous les consentements des patients et/ou de leurs familles pour participer à l’étude ont été recueillis après explication de l’étude.
Selon la classification de la paralysie cérébrale par la SCPE, l’échantillon était composé de 84 % de formes spastiques bilatérales, 16 % de formes choréo-athétosiques ( Tableau 1 ). Sur le plan fonctionnel, parmi ces patients non marchants, 76 % étaient dépendants pour les déplacements en fauteuil roulant et 28 % pouvaient répondre seuls au questionnaire.
| Formes cliniques | |
| PC spastique unilatérale | 0 |
| PC spastique bilatérale | 21 |
| PC Choréo Athétosique | 4 |
| PC Dystonique | 0 |
| PC Ataxique | 0 |
| Topographie de l’atteinte | |
| Quadriplégie | 21 |
| Diplégie | 4 |
| Autonomie de déplacement au fauteuil roulant | |
| Dépendant d’une tierce personne | 19 |
| Autonome | 6 |
2.2.2
Protocole d’implantation
L’indication d’une pompe à BIT était proposée en cas de gêne fonctionnelle secondaire à une spasticité diffuse résistante aux traitements classiques avec un score d’Ashworth modifié supérieur ou égal à 3 . Un test thérapeutique au BIT permettait de valider l’indication de l’implantation d’une pompe à Baclofène en fonction des objectifs fixés avec le patient et sa famille en préthérapeutique et des effets secondaires recueillis. L’objectif thérapeutique antispastique était une diminution d’au moins un point sur l’échelle d’Ashworth. Les objectifs fonctionnels principaux recherchés étaient la facilitation du nursing (toilette, habillage), l’amélioration de l’installation au fauteuil roulant, la diminution des mouvements anormaux (dystoniques ou choréo-athétosiques), l’amélioration de la mobilité (transferts et déplacement en fauteuil) et l’amélioration des douleurs secondaires à la spasticité. Le test était réalisé par ponction lombaire et bolus de 25 à 100 μg de Baclofène ou par cathéter implanté et bolus ou diffusion continue de Baclofène toutes les 24 à 48 heures. Une évaluation de la balance bénéfices/risques permettait d’étudier la tolérance clinique, les doses utilisées, les résultats sur la spasticité six heures après l’injection (échelle d’Ashworth) et les objectifs prédéfinis sur le plan fonctionnel. Si le patient, sa famille et l’équipe médicale et paramédicale étaient satisfaits de l’efficacité des tests par BIT avec une tolérance satisfaisante : l’implantation d’une pompe était programmée.
Après l’implantation, nous avons relevé les changements de posologies à un, trois, six et neuf mois puis de manière annuelle.
Les effets indésirables post-implantation de pompe à Baclofène ont été classés en « complications » en cas de nécessité de réhospitalisation et en « effets secondaires » en cas de traitement symptomatique simple. Le calcul d’incidence a été rapporté en année-pompe, correspondant à une année de traitement par Baclofène.
2.2.3
Évaluation
L’analyse rétrospective des dossiers a recensé les antécédents des patients (comitialité, chirurgies neuro-orthopédiques, escarres), leurs symptomatologies cliniques (douleurs, escarres), leur évaluation préimplantatoire (bilans orthopédiques, bilan de spasticité par l’échelle d’Ashworth modifiée, exploration du rachis). Tous les patients ont été revus entre juin 2008 et juin 2009 pour l’hétéroévaluation de la spasticité par l’échelle d’Ashworth et des conséquences au long cours de l’implantation de la pompe à Baclofène. La situation fonctionnelle a été évaluée suivant le degré d’autonomie de déplacement (dépendance d’une tierce personne ou autonomie au fauteuil roulant) et la fonction cognitive du patient permettant de répondre ou non au questionnaire.
L’évaluation de l’efficacité du BIT a été réalisée de manière subjective par autoquestionnaire par le patient ou sa famille à distance de l’implantation de la pompe à Baclofène. Nous avons utilisé l’échelle visuelle analogique (Eva) de satisfaction compte tenu de sa facilité d’utilisation par des patients présentant des troubles cognitifs ( Annexe I ). Les différents domaines étudiés furent l’évolution des déficiences (douleur, spasticité et facilité d’exécution des mouvements), des limitations d’activité (nursing, soins infirmiers, soins de kinésithérapie, transferts, installation au fauteuil, déplacement en fauteuil roulant, repas, trouble vésicosphinctériens, sommeil), de la qualité de vie ainsi que de la satisfaction globale du BIT. Le questionnaire était rempli par le patient lui-même ou par l’entourage ou les soignants lorsque les troubles cognitifs ne le permettaient pas ( Annexe I ). Les objectifs préthérapeutiques ont été jugés atteints lorsque les patients déclaraient une amélioration supérieure à 5/10 sur l’Eva. Cette évaluation était complétée par un item demandant explicitement si le patient opterait à nouveau pour une pompe à BIT et un espace permettait des commentaires libres.
Compte tenu du faible échantillon de patients et de données rétrospectives sans groupe témoin, les statistiques réalisées pour les données quantitatives sont sous forme de moyennes avec écart-types ou de pourcentages pour l’analyse qualitative des données.
2.3
Résultats
2.3.1
Dispositif technique
Sur le plan technique, toutes les pompes implantées étaient de type Medtronic programmables (24) sauf une à débit continu. La majorité des pompes étaient des Synchromed de type II (16) avec une prédominance de pompes de 40 ml (neuf) ( Fig. 1 ). Le cathéter intrathécal était positionné huit fois en thoracique moyen (32 %), cinq fois en thoracique supérieur (20 %), quatre fois en thoracolombaire (20 %) et dans sept cas, cette donnée n’a pu être précisée.