Imaging is an integral part of the clinical examination of the patient with back pain; it is, however, often used excessively and without consideration of the underlying literature. The primary role of imaging is the identification of systemic disease as a cause of the back or limb pain; magnetic resonance imaging (MRI) excels at this. Systemic disease as a cause of back or limb pain is, however, rare. Most back and radiating limb pain is of benign nature, owing to degenerative phenomena. There is no role for imaging in the initial evaluation of the patient with back pain in the absence of signs or symptoms of systemic disease. When conservative care fails, imaging may be undertaken with due consideration of its risks: labeling the patient as suffering from a degenerative disease, cost, radiation exposure, and provoking unwarranted minimally invasive or surgical intervention. Imaging can well depict disc degeneration and disc herniation. Imaging can suggest the presence of discogenic pain, but the lack of a pathoanatomic gold standard obviates any definitive conclusions. The imaging natural history of disc herniation is resolution. There is very poor correlation between imaging findings of disc herniation and the clinical presentation or course. Psychosocial factors predict functional disability due to disc herniation better than imaging. Imaging with MRI, computed tomography (CT), or CT myelography can readily identify central canal, lateral recess, or foraminal compromise. Only when an imaging finding is concordant with the patient’s pain pattern or neurologic deficit can causation be considered. The zygapophysial (facet) and sacroiliac joint are thought to be responsible for axial back pain, although with less frequency than the disc. Imaging findings of the structural changes of osteoarthritis do not correlate with pain production. Physiologic imaging, either with single-photon emission CT bone scan, heavily T2-weighted MRI sequences (short-tau inversion recovery), or gadolinium enhancement, can detect inflammation and are more predictive of an axial pain generator.
Imaging is a single, though integral, part of the evaluation of the patient with back pain. The comprehensive evaluation must include historical data, the physical examination, and may extend to electrophysiologic tests, imaging, and response to minimally invasive interventions. Imaging can only be appropriately interpreted in the context of the totality of the evaluation; it does not stand alone. To an imaging professional, the ability of modern imaging technology to record an exquisite and accurate representation of patient anatomy and pathology is often believed to have inherent worth. To the clinician and patient, the consumers of imaging, technologically sophisticated imaging only provides value where it advances the diagnosis, excludes sinister disease, or identifies opportunities for evidence-based therapeutic intervention.
Back pain is ubiquitous in Western societies. It is the most common and expensive cause of work disability in the United States. The use of sophisticated imaging in the evaluation of back pain is increasing. Lumbar spine magnetic resonance imaging (MRI), as measured by Medicare use statistics, increased by 307% in the 12-year interval from 1994 to 2005. Despite the greater intensity of evaluation, there is no evidence that patient outcomes have improved. Rather, much of spine imaging is often unreasoned and adds no value to the patient’s evaluation. There are very large regional variations in the intensity of spine imaging across United States; from one-third to two-thirds of spine computed tomography (CT) and MRI studies are judged to be inappropriate when measured against established guidelines. It is hoped that this review of the imaging literature will provide a more rational basis for the use of imaging in patients with back or leg pain.
When considering the use of sophisticated imaging technologies in the patient with back or leg pain, it must be remembered that this occurs in the context of overwhelmingly benign disease, in terms of both the underlying pathophysiology and the clinical course. Back pain is most commonly clinically benign and self-limiting, and will benefit neither from imaging evaluation nor invasive therapies. Von Korff and colleagues studied patients with a recent history of low back pain; at 6 months from onset, 76% of patients either had no pain (21%) or mild pain and disability, and 14% had significant disability with moderate to severe limitation of function. Another study noted that whereas 70% of patients with acute low back pain have persistent pain at 4 weeks from onset, at 12 weeks only 35% experience persistent discomfort and at 1 year only 10% have persistent pain. In an Australian study consisting of 2 populations, 49% to 67% had recovered from low back pain at 3 months following onset, and at 12 months 56% to 71% had recovered. There was a relapse rate of 7% to 27% within 1 year. Although these recent studies illustrate that the clinical prognosis of low back pain is not as positive as had been thought a generation ago, the vast majority of patients will indeed recover. Many of these patients have musculoskeletal strains or sprains, or suffer from nonspecific degenerative phenomena that may elude a clear diagnosis in up to 85% of patients.
In terms of underlying pathophysiology, back or leg pain is overwhelmingly caused by benign disease. A differential diagnosis of low back pain with the associated prevalence of underlying pathologic processes has been compiled by Jarvik and Deyo and is presented in Table 1 . Their analysis suggests that 95% of low back pain is due to benign processes. In patients presenting to a primary care setting with low back pain, only 0.7% will suffer from undiagnosed metastatic neoplasm. Spine infection, including pyogenic and granulomatous discitis, epidural abscess, or viral processes, will be present in only 0.01% of subjects. Noninfectious inflammatory spondyloarthropathies, such as ankylosing spondylitis, will account for 0.3% of presentations. Osteoporotic compression fractures are the most common systemic pathologic process to present as back pain, accounting for 4% of patients.
| Mechanical Low Back or Leg Pain (97%) | Nonmechanical Spine Conditions (1%) | Visceral Disease (2%) |
|---|---|---|
| Lumbar strain or sprain (70%) Degenerative process of disc and facets (usually related to age) (10%) Herniated disc (4%) Spinal stenosis (3%) Osteoporotic compression fracture (4%) Spondylolisthesis (2%) Traumatic fractures (<1%) Congenital disease (<1%) Severe kyphosis Severe scoliosis Transitional vertebrae Spondylolysis Internal disc disruption or discogenic back pain Presumed instability | Neoplasia (0.7%) Multiple myeloma Metastatic carcinoma Lymphoma and leukemia Spinal cord tumors Retroperitoneal tumors Primary vertebral tumors Infection (0.01%) Osteomyelitis Septic discitis Paraspinous abscess Epidural abscess Shingles Inflammatory arthritis (often HLA-B27 associated) (0.3%) Ankylosing spondylitis Psoriatic spondylitis Reiter syndrome Inflammatory bowel disease Scheuermann disease (osteochondrosis) Paget disease | Pelvic organ involvement Prostatitis Endometriosis Chronic pelvic inflammatory disease Renal involvement Nephrolithiasis Pyelonephritis Perinephric abscess Aortic aneurysm Gastrointestinal involvement Pancreatitis Cholecystitis Penetrating ulcer |
The primary role of imaging is to identify the approximately 5% of patients with back pain who have undiagnosed systemic disease as the cause of their pain. A related imaging goal is to characterize and assist in therapy planning in the very small percentage of patients who have neural compressive disease resulting in radiculopathy or radicular pain syndromes that fail conservative therapy and require surgical or minimally invasive intervention. Imaging identification of degenerative disease in the patient with back pain is, as this article elaborates, seldom helpful and is at best of secondary concern.
Early imaging
This review first addresses the utility of imaging in a patient who presents acutely with back or leg pain. It is well established that there is no role for imaging in this patient population in the absence of information that would suggest underlying systemic disease or signs of neurologic impairment which may require intervention; this applies to both radiographs and advanced imaging. A study by Scavone and colleagues evaluated spine radiographs in patients who present with acute low back pain; 75% of the studies provided no useful information. A United Kingdom study randomized patients who had experienced back pain for 6 weeks to further clinically guided care or lumbar radiographs. At 9 months’ follow-up there were no significant differences in clinical outcomes. Gilbert and colleagues randomized patients between advanced imaging (CT or MRI) at presentation with back pain versus imaging only when a clear clinical indication developed. Early advanced imaging had no significant effect on patient outcomes. Chou and colleagues recently performed a meta-analysis of all randomized controlled trials comparing immediate imaging (radiographs, CT, MRI) with clinically directed care in acute patients with back pain. In the pooled data from the 6 qualifying trials, analysis showed no significant differences in pain or function in imaged versus nonimaged patients in either the short term (3 months) or long-term (6–12 months). Chou and colleagues concluded “lumbar imaging for low back pain without indications of serious underlying conditions does not improve clinical outcomes.”
In addition to being ineffective, early employment of imaging is costly; a cost-effectiveness analysis by Liang and Komaroff showed that simply performing radiographs at the initial presentation of back pain results in a cost of $2000 (1982 dollars) to alleviate a single day of pain. Carragee and colleagues elegantly demonstrated the lack of utility of imaging in the acute setting in a 5-year prospective observational study. A large cohort of asymptomatic subjects deemed to be at risk for back pain resulting from labor-intensive vocations underwent lumbar spine MRI. This patient cohort was followed periodically over the next 5 years; a subset of these subjects presented to a medical care provider with acute back or leg pain during this 5-year period and a second lumbar MRI was obtained. Less than 5% of the MRI scans obtained at the time of acute presentation with back or leg pain showed clinically relevant new findings; virtually all of the “positive findings” noted on the images at the time of presentation with back/leg pain had been present on the baseline studies obtained when the patient was asymptomatic. Only direct evidence of neural compression in patients with a corresponding radicular pain syndrome was considered to be useful imaging information. Of particular note, psychosocial factors, not the morphology seen on imaging, were the best predictors of the degree of functional disability caused by back/leg pain.
Based on such data, numerous professional organizations and societies have issued guidelines that recommend against imaging early in a clinical pain syndrome. In 1994, the Agency for Health Care Policy and Research recommended against imaging patients with back pain within the first month of a pain syndrome in the absence of signs of systemic disease. The American College of Radiology practice guidelines were recently restated by Bradley in 2007. Imaging the patient who presents with acute low back pain is not indicated except in the presence of “red flag” features including recent significant trauma, minor trauma in a patient older than 50 years, weight loss, fever, immunosuppression, history of neoplasm, steroid use or osteoporosis, age greater than 70 years, known intravenous drug abuse, or a progressive neurologic deficit with intractable symptoms. Similarly, a joint recommendation from the American College of Physicians and the American Pain Society stated that imaging should not be obtained in patients with nonspecific low back pain. Imaging should only be performed when severe or progressive neurologic deficits are present or when serious underlying systemic disease is suspected. Furthermore, patients with signs or symptoms of radiculopathy or spinal stenosis should be imaged only if they are candidates for surgical or minimally invasive intervention (eg, epidural steroid injection). These recommendations further reinforce the primary role of imaging as a means of detecting underlying systemic disease, most commonly neoplasm, infection, or unsuspected traumatic injury.
Risk/benefit analysis
In a patient who has failed conservative therapy or who has “red flag” features that suggest underlying systemic disease, the clinician may decide to begin imaging. Such a decision must be a rational balance of risk and benefit. Certainly there are benefits to be derived from imaging. Foremost, imaging may suggest, and assist in, the diagnosis of, previously unsuspected systemic disease. In the patient with a radicular pain syndrome or radiculopathy that has not responded to conservative therapy, imaging may supply invaluable information that allows planning of minimally invasive or surgical procedures. Negative imaging should also have value, in providing reassurance that there is no sinister disease present and in stopping further workup in appropriate circumstances. Finally, in patients with chronic pain syndromes, imaging may assist in the identification of the structural cause of such pain. Only when a specific pain generator is identified can a specific plan of therapeutic intervention, whether it be conservative or invasive, be developed.
Risk, the inevitable counterweight to benefit, is often not considered in the decision to undertake imaging. Imaging does carry risks; these include the labeling effect, radiation exposure, cost, and the provocation of intervention.
The labeling effect refers to the inevitable presence of degenerative findings on any imaging study; the subject now becomes a patient carrying a diagnosis of degenerative spine disease. The word “degenerative” carries only negative connotations. This negativity is illustrated in a study performed by Modic and colleagues, in which patients who presented with back pain underwent MRI but were randomized to either disclosure of the MRI findings to the patient and physician, or withholding such information. Among the findings in this study, patients who received the MRI findings of benign degenerative phenomena actually had a diminished sense of well-being when compared with subjects from whom the MRI report was withheld. This speaks to the critical need for physicians to communicate to the patient the lack of significance of the vast majority of degenerative findings identified on imaging. Unless appropriately educated to the contrary, patients may perceive this as representing the start of an inevitable downward spiral of spine degeneration, which may lead to fear avoidance behaviors with diminished activity, deconditioning, and depression. A recent Cochrane database review established the effectiveness of active patient education, particularly in the setting of acute low back pain. The irrelevance of degenerative findings on imaging studies, and the importance of maintaining functional strength and high activity levels, must be reinforced at every patient encounter.
Radiation exposure from radiographs, CT, and nuclear medicine studies carries a cumulative risk of neoplasm induction. This risk becomes particularly problematic when serial studies are performed. The biologically effective absorbed radiation dose is measured by the Sievert (Sv); in North America, the average annual natural background exposure is approximately 3 mSv. A frontal and lateral chest radiograph is often considered the common currency of radiation exposure, incurring a dose of approximately 0.1 mSv. A 3-view lumbar spine radiographic series is then worth approximately 15 chest radiographs, or 1.5 mSv. A dose of 6 mSv is typical for a lumbar spine CT scan, a value of 60 chest radiographs. Similarly, a technetium bone scan has a dose of 6.3 mSv. In this context, an abdomen and pelvis CT study will incur 14 mSv on average. All radiation exposure accumulates over the patient’s lifetime and contributes to a risk of radiation induced cancer. Imaging studies that use radiation must be employed with careful consideration of the risk and anticipated benefits.
Imaging is costly. In the United States the medical imaging community incurs more than $100 billion of societal cost per year. The 2009 Medicare reimbursements for lumbar spine imaging include radiographs: $41; noncontrast CT: $264; myelogram: $506; noncontrast MRI: $439; whole body positron emission tomography (PET)/CT: $1183; bone scan with single-photon emission CT (SPECT): $261. Nominal fees are typically 3 to 5 times the Medicare reimbursements. It is easy to appreciate how quickly imaging costs can accrue.
A less frequently considered risk of imaging is the provocation of intervention. Jarvik and colleagues documented that obtaining advanced imaging (MRI) early in a patient’s spine pain syndrome leads to increased surgical interventions despite equivalent pain and disability profiles, when compared with nonimaged patients. Likewise, Lurie and colleagues examined the dramatic regional variation (12-fold) in the rate of surgical intervention for central canal stenosis. These investigators noted that the rate of surgical intervention correlated directly with the intensity of CT and MRI use. When advanced imaging shows an “abnormality” potentially responsible for a patient’s pain, the temptation is to correct that imaging finding. Such a situation occurs despite the well-established lack of specificity of many of the spine imaging “abnormalities,” which are discussed shortly. It is critical to treat the patient, not the image.
Risk/benefit analysis
In a patient who has failed conservative therapy or who has “red flag” features that suggest underlying systemic disease, the clinician may decide to begin imaging. Such a decision must be a rational balance of risk and benefit. Certainly there are benefits to be derived from imaging. Foremost, imaging may suggest, and assist in, the diagnosis of, previously unsuspected systemic disease. In the patient with a radicular pain syndrome or radiculopathy that has not responded to conservative therapy, imaging may supply invaluable information that allows planning of minimally invasive or surgical procedures. Negative imaging should also have value, in providing reassurance that there is no sinister disease present and in stopping further workup in appropriate circumstances. Finally, in patients with chronic pain syndromes, imaging may assist in the identification of the structural cause of such pain. Only when a specific pain generator is identified can a specific plan of therapeutic intervention, whether it be conservative or invasive, be developed.
Risk, the inevitable counterweight to benefit, is often not considered in the decision to undertake imaging. Imaging does carry risks; these include the labeling effect, radiation exposure, cost, and the provocation of intervention.
The labeling effect refers to the inevitable presence of degenerative findings on any imaging study; the subject now becomes a patient carrying a diagnosis of degenerative spine disease. The word “degenerative” carries only negative connotations. This negativity is illustrated in a study performed by Modic and colleagues, in which patients who presented with back pain underwent MRI but were randomized to either disclosure of the MRI findings to the patient and physician, or withholding such information. Among the findings in this study, patients who received the MRI findings of benign degenerative phenomena actually had a diminished sense of well-being when compared with subjects from whom the MRI report was withheld. This speaks to the critical need for physicians to communicate to the patient the lack of significance of the vast majority of degenerative findings identified on imaging. Unless appropriately educated to the contrary, patients may perceive this as representing the start of an inevitable downward spiral of spine degeneration, which may lead to fear avoidance behaviors with diminished activity, deconditioning, and depression. A recent Cochrane database review established the effectiveness of active patient education, particularly in the setting of acute low back pain. The irrelevance of degenerative findings on imaging studies, and the importance of maintaining functional strength and high activity levels, must be reinforced at every patient encounter.
Radiation exposure from radiographs, CT, and nuclear medicine studies carries a cumulative risk of neoplasm induction. This risk becomes particularly problematic when serial studies are performed. The biologically effective absorbed radiation dose is measured by the Sievert (Sv); in North America, the average annual natural background exposure is approximately 3 mSv. A frontal and lateral chest radiograph is often considered the common currency of radiation exposure, incurring a dose of approximately 0.1 mSv. A 3-view lumbar spine radiographic series is then worth approximately 15 chest radiographs, or 1.5 mSv. A dose of 6 mSv is typical for a lumbar spine CT scan, a value of 60 chest radiographs. Similarly, a technetium bone scan has a dose of 6.3 mSv. In this context, an abdomen and pelvis CT study will incur 14 mSv on average. All radiation exposure accumulates over the patient’s lifetime and contributes to a risk of radiation induced cancer. Imaging studies that use radiation must be employed with careful consideration of the risk and anticipated benefits.
Imaging is costly. In the United States the medical imaging community incurs more than $100 billion of societal cost per year. The 2009 Medicare reimbursements for lumbar spine imaging include radiographs: $41; noncontrast CT: $264; myelogram: $506; noncontrast MRI: $439; whole body positron emission tomography (PET)/CT: $1183; bone scan with single-photon emission CT (SPECT): $261. Nominal fees are typically 3 to 5 times the Medicare reimbursements. It is easy to appreciate how quickly imaging costs can accrue.
A less frequently considered risk of imaging is the provocation of intervention. Jarvik and colleagues documented that obtaining advanced imaging (MRI) early in a patient’s spine pain syndrome leads to increased surgical interventions despite equivalent pain and disability profiles, when compared with nonimaged patients. Likewise, Lurie and colleagues examined the dramatic regional variation (12-fold) in the rate of surgical intervention for central canal stenosis. These investigators noted that the rate of surgical intervention correlated directly with the intensity of CT and MRI use. When advanced imaging shows an “abnormality” potentially responsible for a patient’s pain, the temptation is to correct that imaging finding. Such a situation occurs despite the well-established lack of specificity of many of the spine imaging “abnormalities,” which are discussed shortly. It is critical to treat the patient, not the image.
Specificity, sensitivity, and reliability
Having considered risk and benefit, it is also imperative to know the reliability of imaging findings, as well as shortcomings in sensitivity and specificity that plague all spine imaging. Table 2 , compiled by Jarvik and Deyo, describes the estimated accuracy of several imaging techniques for various lumbar spine conditions. Although the primary role of imaging is the detection of underlying systemic disease, the low prevalence of systemic disease as a cause of back pain implies most imaging studies will primarily describe degenerative phenomena. Degenerative changes are ubiquitous on imaging studies, seldom causal of an individual patient’s pain syndrome, and often inappropriately considered as pain generators, precipitating unnecessary interventions.
| Technique | Sensitivity | Specificity | Positive Likelihood Ratio | Negative Likelihood Ratio |
|---|---|---|---|---|
| Plain Radiography | ||||
| Cancer | 0.6 | 0.95–0.995 | 12–120 | 0.40–0.42 |
| Infection | 8.82 | 0.57 | 1.9 | 0.32 |
| Ankylosing spondylitis | 0.26–0.45 | 1 | ND | 0.55–0.74 |
| Computed Tomography | ||||
| Herniated disc | 0.62–0.9 | 0.7–0.87 | 2.1–6.9 | 0.44–0.54 |
| Stenosis | 0.9 | 0.8–0.95 | 4.5–22 | 0.10–0.12 |
| Magnetic Resonance Imaging | ||||
| Cancer | 0.83–0.93 | 0.90–0.97 | 8.3–31 | 0.07–0.19 |
| Infection | 0.96 | 0.92 | 12 | 0.04 |
| Ankylosing spondylitis | 0.56 | 0.43–0.97 | ||
| Herniated disc | 0.6–1.0 | 0.72–1.0 | 1.1–33 | 0–0.93 |
| Stenosis | 0.9 | 3.2–ND | 0.10–0.14 | |
| Radionuclide Scanning | ||||
| Cancer | ||||
| Planar imaging | 0.74–0.98 | 0.64–0.83 | 3.9 | 0.32 |
| SPECT | 0.87–0.93 | 0.91–0.93 | 9.7 | 0.14 |
| Infection | 0.9 | 0.78 | 4.1 | 0.13 |
| Ankylosing spondylitis | 0.26 | 1 | ND | 0.74 |
a Estimated ranges are derived from multiple studies described in the text.
The observation that there is a high prevalence of asymptomatic degenerative changes in the spine is not new. Hult studied adults in 1954 and showed that by age 50 years, 87% will have radiographic evidence of disc degeneration (narrowing of the disc space, marginal sclerosis with osteophytes, vacuum phenomena). In a second study including a cohort of asymptomatic workers, Hult noted radiographic evidence of disc disease in 56% of those aged 40 to 44 years, which rose to 95% in subjects 50 to 59 years old. With the evolution of more sophisticated spine imaging techniques, this lack of specificity of degenerative findings has not improved. Hitselberger and Witten studied plain myelography of asymptomatic volunteers and noted that 24% showed abnormalities that would have been considered significant in a clinical context of back or leg pain. A study of lumbar spine CT in asymptomatic volunteers by Wiesel and colleagues showed that in patients older than 40 years, 50% had “significant” abnormalities. Similarly, Boden and colleagues evaluated MRI of the lumbar spine in asymptomatic volunteers; in patients older than 60 years, 57% had abnormalities that would have been considered significant in an appropriate clinical setting. Jarvik and colleagues studied a large patient population with MRI. This study noted that only extrusions, moderate to severe central canal stenosis, and direct visualization of neural compression were likely to be significant and would separate patients with pain from asymptomatic volunteers. Disc protrusions, zygapophysial joint (z-joint) arthropathy, and antero- or retrolisthesis were virtually always asymptomatic findings. Imaging studies of asymptomatic volunteers are compiled in Table 3 .
| Test | Study Ref , Year | Patients (N) | Age Range (Mean) | Disc Herniation | Disc Bulge | Disc Degeneration | Central Canal Stenosis | Annular Fissure |
|---|---|---|---|---|---|---|---|---|
| Radiograph | Hult, 1954 | 1200 | 40–44 55–59 | 56% 95% | ||||
| Radiograph | Hellstrom et al, 1990 | 143 | 14–25 | 20% | ||||
| Myelogram | Hitselberger and Witten, 1968 | 300 | [51] | 31% | ||||
| CT | Wiesel et al, 1984 | 51 | [40] | 20% | 3.40% | |||
| MRI | Weinreb et al, 1989 | 86 | [28] | 9% | 44% | |||
| MRI | Boden et al, 1990 | 53 | <60 ≥60 | 22% 36% | 54% 79% | 46% 93% | 1% 21% | |
| MRI | Jensen et al, 1994 | 98 | [42] | 28% | 52% | 7% | ||
| MRI | Boos et al, 1995 | 46 | [36] | 76% | 51% | 85% | ||
| MRI | Stadnik et al, 1998 | 36 | [42] | 33% | 81% | 56% | 56% | |
| MRI | Weishaupt et al, 1998 | 60 | [35] | 60% | 28% | 72% | 20% | |
| MRI | Jarvik et al, 2001 | 148 | [54] | 38% | 64% | 91% | 10% | 38% |
More recent studies have addressed the prevalence of degenerative imaging findings in younger populations, primarily in Scandinavian countries; these are MRI population-based studies without regard to symptomatology. Kjaer and colleagues, studying children age 13 years, found a 21% prevalence of disc degeneration. In a study of adolescents, Salminen and colleagues found a 31% prevalence of disc degeneration in 15-year-olds, which rose to 42% in 18-year-olds. Takatalo and colleagues evaluated 558 young adults aged 20 to 22 years. Using the 5-point Pfirrmann classification of disc degeneration, they noted disc degeneration of grade 3 or higher in 47% of these young adults. There was a higher prevalence in males (54%) than in females (42%). Multilevel degeneration was identified in 17%.
The lack of specificity of degenerative imaging findings is clear. Population-based studies show a prevalence of imaging degenerative findings greatly in excess of the symptomatic disease prevalence. Studies of asymptomatic cohorts show a high prevalence of asymptomatic degenerative imaging findings. In general, one-third to two-thirds of asymptomatic subjects will exhibit degenerative findings on all imaging studies; the prevalence of asymptomatic degenerative findings increases with increasing age. Disc bulges, protrusions, facet arthropathy, and antero- or retrolisthesis are common, usually asymptomatic, and their prevalence increases with increasing age. Disc extrusions, severe central canal stenosis, and direct evidence of neural compression are more likely to be truly symptomatic imaging findings. In the individual patient, only the clear concordance of a clinical pain syndrome and imaging can suggest causation. Even in this setting, anesthetic or provocative tests may be needed to allow rational decision making, particularly where interventions of significant risk and cost are contemplated.
On the flip side of the specificity coin, spine imaging also has a basic sensitivity flaw. The literature documents well that neuroclaudicatory pain is exacerbated by extension positioning and axial loading. Schmid and colleagues demonstrated that the cross-sectional area of the lumbar central spinal canal, its lateral recesses, and neural foramina all diminish when going from neutral to extension positioning and with the assumption of axial load (standing vs recumbent). Danielson and Willen looked at cohorts of asymptomatic volunteers and patients with neuroclaudicatory pain syndromes. These investigators noted that axial loading and extension would reduce the cross-sectional dimension of the dural sac in 50% of asymptomatic patients, but in 76% to 80% of patients who had neuroclaudicatory pain. The vast majority of advanced imaging (CT, MRI) is performed with the subject in a supine, psoas relaxed position with the legs slightly elevated, which is done for patient comfort and to minimize motion during the course of the study. This loss of normal lumbar lordosis as well as lack of axial load diminishes sensitivity to the detection of dynamic neural compressive lesions. There is significant enthusiasm in the literature for more physiologic imaging, sometimes referred to as P/K (Positional/Kinetic) or upright MRI. Existing scanners that allow weight-bearing and dynamic studies are of low field strength, 0.6 T. The lower magnetic field results in a lower signal to noise ratio and ultimately lower imaging quality. Patients also find it problematic to hold a position in which their pain is exacerbated, with resultant motion artifact. Loading devices that apply a compressive load and extension positioning in the lumbar region can be used on conventional high-field MRI scanners or CT; this is certainly much more economically palatable. Studies by Danielson and Willen suggest that loading the patient to 50% of the body weight for 5 minutes with subsequent imaging is a reasonable reproduction of upright imaging. Such devices do not allow more complex motions such as rotation or side bending.
The reliability of MRI in detecting and describing degenerative phenomena in the lumbar spine has been best and most comprehensively studied using the data from the Spine Patient Outcomes Research Trial (SPORT). Lurie and colleagues studied the reliability of MRI in the detection and categorization of disc herniations, the degree of thecal sac compromise, and the grading of nerve root impingement. Interreader reliability was substantial for disc morphology (herniation), moderate for the degree of thecal sac compression, and moderate for grading nerve route impingement. Nondisc contour degenerative findings were subsequently studied by Carrino and colleagues ; they noted good interobserver agreement in rating the degree of disc degeneration. There was moderate interobserver agreement in the rating of spondylolisthesis, Modic endplate changes, z-joint arthropathy, and annular high-intensity zones (HIZ).
Imaging modalities
Having weighed the risks and benefits of imaging, and acknowledged its shortcomings in specificity and sensitivity, the clinician may proceed with imaging, which should begin with radiographs. This procedure will typically occur in the setting of clinical “red flags,” pain unresponsive to conservative care, or progressive neurologic deficit. Weight-bearing radiographs provide essential information on vertebral enumeration and coronal and sagittal alignment, which cannot be obtained with more advanced modalities. Vertebral enumeration should not be trivialized. Up to 12% of the general population will have anomalies of segmentation (transitional segments) at the lumbosacral junction. Radiographs will establish vertebral numbering for subsequent advanced imaging and intervention; this will diminish the likelihood of wrong-level interventions at a later date. Radiographs also serve as a low-sensitivity screening tool for evidence of neoplasm, fracture, or infection. Degenerative findings, including disc space narrowing, z-joint arthropathy, and minor degrees of antero- or retrolisthesis are likely insignificant and unrelated to an individual patient’s pain syndrome. Single frontal and lateral standing images of the lumbar spine are adequate. Oblique views double the gonadal radiation dose and should not be obtained routinely. Flexion-extension or side bending views should only be obtained in an operative planning setting.
When radiographs are not explanatory of an unremitting pain syndrome or suggest underlying systemic disease, advanced imaging (CT, MRI, nuclear medicine) is obtained. CT has undergone a revolution in the last decade with the advancement of multidetector technology. A dataset for the lumbar spine can now be obtained in a few seconds, eliminating motion artifact and dramatically improving patient tolerance. This dataset can then be reconstructed in any plane without loss of spatial resolution or additional radiation exposure.
CT provides superior imaging of cortical and trabecular bone when compared with MRI. For this reason, CT may be necessary to characterize primary bone tumors of the spine. CT also provides reasonable contrast resolution and can identify root compressive lesions such as disc herniations in the vast majority of cases. CT cannot identify intrathecal pathology and is less sensitive than MRI in the detection of early inflammatory or infectious processes, neoplasm, or paraspinal soft tissue lesions. Radiation dose must always be considered when employing CT. One by-product of the rapid recent technological advance of CT is that the literature contains no comparative studies between MRI and the current generation of multidetector CT scanners in the detection and characterization of disc herniations.
MRI has been the dominant spine imaging modality for the past 2 decades, despite relatively little technological advancement in that time span. MRI has superior contrast resolution and thus the ability to distinguish between soft tissue types, allowing it to detect intrathecal pathology and identify subtle root compressive lesions. MRI has superior sensitivity in the detection of neoplasm and infection. With the use of gadolinium contrast, or heavily T2-weighted imaging sequences (short-tau inversion recovery [STIR] or fast spin echo T2 sequences with fat saturation), MRI can detect inflammatory change. It has greater specificity than CT in characterizing the chronicity of fractures. With gadolinium enhancement, MRI can distinguish between recurrent disc herniation and scarring in the postoperative patient. MRI does not evaluate cortical bone well.
Patient acceptance remains problematic, with high cost, prolonged imaging times, and up to 10% examination failures due to claustrophobia. Open magnets have improved patient acceptance, but at the cost of image quality. A small percentage of patients are MRI incompatible due to pacemakers, spinal cord stimulators, or other implanted devices.
CT myelography retains a problem-solving role in the lumbar spine; it will substitute for MRI in the incompatible patient. CT myelography has superior spatial resolution when compared with MRI, but lacks its soft tissue contrast resolution. With the addition of intrathecal contrast material, it can provide exquisite demonstration of root compressive lesions and central canal, lateral recess, and foraminal compromise. CT myelography is minimally invasive, expensive, operator dependent to a degree, and also requires current CT technology to be maximally useful.
Nuclear medicine studies are growing in importance in spine imaging. Technetium bone scans detect accelerated bone metabolic activity. With the addition of SPECT capability, significant additional spatial resolution is possible. Such imaging is useful in assessing the burden of metastatic disease and also in the evaluation of patients with clinically suspected spondylolysis. When MRI is not technically feasible, technetium bone scanning can be used to characterize the chronicity of vertebral fractures in selecting patients for bone augmentation. Bone scans can identify noninfectious inflammatory disease in the facet and sacroiliac joints, aiding in the identification of pain generators and targeting these structures for injection. The technetium bone scan in combination with gallium scan offers sensitivity equal to MRI in the detection of spondylodiscitis. However, they do provide less anatomic information and MRI may ultimately be necessary to characterize the degree of central canal compromise that may influence surgical decision making. PET or PET/CT scans have an increasing role in assessing the burden of metastatic disease and in selecting lesions for percutaneous biopsy.
Imaging of degenerative phenomena
Although the primary role of imaging in the patient with back/leg pain is the detection of systemic disease, or characterization of neural compressive disease requiring intervention, the low prevalence of these processes dictates that most spine imaging will inevitably be used to evaluate degenerative disease. This section initially discusses imaging of degenerative processes, followed by imaging findings in systemic disease that may present as back or leg pain. Disc degeneration is a multifactorial process with genetic, inflammatory, traumatic, and nutritional components. It is beyond the scope of this article to deal with disc degeneration in depth, and this is covered in the article by Wolfer elsewhere in this issue.
Disc degeneration is manifest on plain radiographs as loss of disc space height, nitrogen gas within the disc space (vacuum sign), and marginal osteophytes. In the normal adult, disc space height increases as one proceeds caudally from L1 through L4; the L5 or lumbosacral disc is variable in height and may normally be less than that of L4. On CT images, particularly sagittal reconstructions, findings of disc degeneration are identical to those seen on radiographs, although detected with greater sensitivity. On T2-weighted MRI images, the normal nuclear compartment of the disc is hyperintense, bounded by the hypointense annulus. The intranuclear cleft, a horizontal hypointense band crossing the center of the nucleus, is a normal finding in the mature disc. In early disc degeneration the intranuclear cleft is lost, and the junction between the hyperintense nucleus and the low signal annulus becomes indistinct ( Fig. 1 A–D). This diminished nuclear signal reflects loss of proteoglycans and alteration in their hydration state; it is not simply dehydration. There may be subsequent loss of disc space height. Failure of the fibrous annulus results in fissuring, which may be detected as a focal zone of elevated T2 signal (HIZ) or gadolinium enhancement within the outer annulus. The outer third of the annulus is known to be innervated, with afferent supply via the sinuvertebral nerve. As fissures penetrate the annulus to the epidural space, granulation tissue will invade the disc, with ingrowth of unmyelinated C-type nociceptors into the inner annulus or nuclear compartment. Exposure to the nuclear biochemical environment is postulated to sensitize these nociceptors, such that they fire at very low levels of mechanical stress, including those experienced in activities of daily living; this is thought to be the genesis of the sitting and standing intolerance of discogenic pain. Potential imaging markers of discogenic pain are now discussed. Discography as a diagnostic test is discussed in the article by Alison Stout elsewhere; its utility remains controversial.
Endplate Changes
The functional unity of the disc and the cartilaginous endplate is apparent in signal changes within the endplate and adjacent subchondral marrow that accompany disc degeneration. Endplate marrow changes were originally classified by Modic and colleagues in 1988 ( Fig. 2 A–F). Type I change represents ingrowth of vascularized granulation tissue into sub-endplate marrow; it exhibits hypointense T1 and hyperintensity T2 signal on MRI and may enhance with gadolinium. Type II change exhibits elevated T1 and T2 signal and reflects fatty infiltration of sub-endplate marrow. Type III change is hypointense on T1 and T2; it correlates with bony sclerosis. Type I change is thought to represent an active inflammatory state, with type II being more quiescent, and type III postinflammatory. Ohtori and colleagues noted elevated levels of protein gene product (PGP) 9.5 immunoreactive nerve fibers and tumor necrosis factor (TNF) immunoreactive cells in the cartilaginous endplates of patients with Modic changes. The immunoreactive nerve ingrowth was seen exclusively in patients with discogenic low back pain. TNF immunoreactive cells were more common in type I endplate changes.
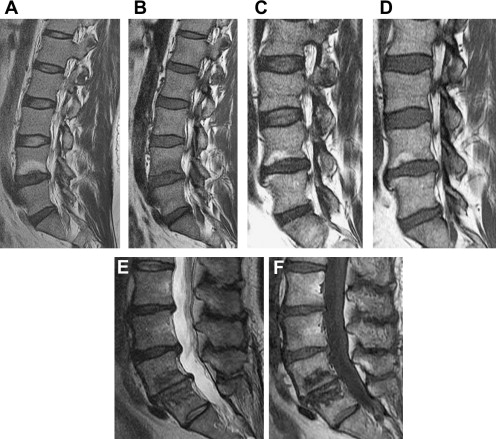
Modic endplate changes do carry an association with low back pain, particularly type I change. Toyone and colleagues found 73% of patients with type I change had low back pain as opposed to 11% with type II change. Likewise, Albert and Manniche reported low back pain in 60% of patients with Modic changes but in only 20% for those without Modic change. Type I change was more strongly associated with low back pain than type II change. Several investigators (Braithwaite and colleagues, Ito and colleagues, Weishaupt and colleagues, Lei and colleagues, O’Neill and colleagues, and Kang and colleagues ) have correlated the response to provocation discography with type I and type II endplate changes. These studies consistently show a very high specificity (87%–98%) but a relatively low sensitivity (14%–48%) for type I and/or type II endplate change as a predictor of discogenic pain as defined by provocation discography. These studies either did not discriminate between type I and type II changes, or when differential data were available, did not show a great advantage for type I change as a predictor of discogenic pain.
Modic type I change may also be associated with segmental instability. In the study of Toyone and colleagues, 70% of patients with type I change were found to have segmental hypermobility (>3 mm translation on flexion-extension films). Hypermobility was seen in only 16% of those with type II change. Similarly, in post fusion patients, the studies of Butterman and colleagues and Lang and colleagues showed persistent type I change in patients ultimately shown to have pseudoarthroses. Patients with solid fusions tend to have type II change or resolution of all Modic changes. The studies of Chataigner and colleagues and Esposito and colleagues evaluated Modic change as a predictor of fusion outcome. Patients with type I change at the operative level on preprocedure imaging tended to have much better outcomes than patients operated on with isolated disc degeneration or disc degeneration plus type II endplate change.
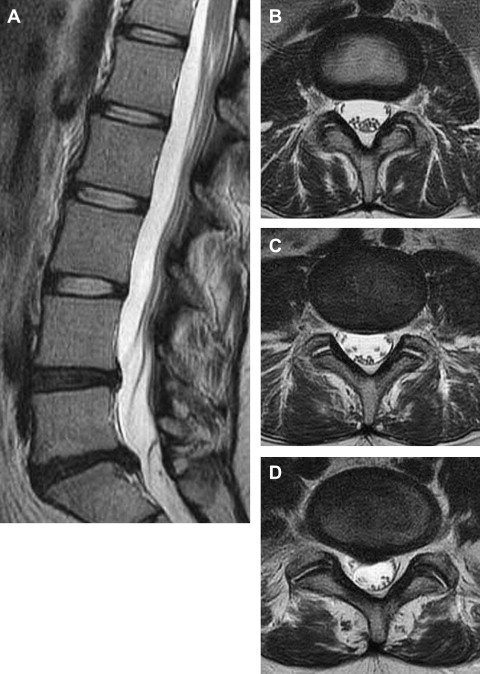
Annular Fissure (HIZ)
In 1992, Aprill and Bogduk described the HIZ, a focus of bright T2 signal within the posterior annulus, as an MRI marker of a painful degenerated disc ( Fig. 3 A–E). Pathologically, this represents an annular fissure; it will typically enhance with gadolinium, indicating the presence of vascularized granulation tissue. Using exact pain reproduction at provocation discography as diagnostic of discogenic pain, the presence of an HIZ had a specificity of 89% and a sensitivity of 82%. In other consecutive series (Carragee and colleagues, Ricketson and colleagues, Smith and colleagues, Ito and colleagues, Weishaupt and colleagues, Kang and colleagues, O’Neil and colleagues ), the high specificity of the HIZ for discogenic pain as identified by discography has been consistently demonstrated (84%–93%). The prevalence of the HIZ finding (per disc) in these series ranged from 9% to 30%; the sensitivity spanned 11% to 57%. The HIZ is a highly specific but somewhat insensitive indicator of a painful disc.






