How Do We Prevent the Transition from Acute to Chronic Pain After Whiplash Injury?
Michele Sterling
Whiplash (WL) injury following a road traffic crash is common, with recent figures suggesting more than 300 persons (per 100,000 in the population) are seen in emergency departments every year in Europe and North America [7], and in Australia, WL injuries comprise ˜75% of all survivable road traffic crash injuries [9]. Musculoskeletal conditions and injuries from road traffic crashes account for a large proportion of disease burden worldwide, with the burden associated with such conditions increasing [67]. WL injury incurs substantial economic costs, exceeding $350 million in Queensland, Australia from 2011 to 2012 [42]; in excess of £3 billion per year in the United Kingdom [31], and $230 billion US dollars per annum in 2000 in the United States [3].
Consistent international data indicate that approximately 50% of people who sustain a WL injury will not recover but will continue to report ongoing pain and disability 1 year after the injury [7]. Mental health outcomes are also poor, with the prevalence of psychiatric disorders in people with persistent whiplash-associated disorders (WAD) around 25% for posttraumatic stress disorder (PTSD) [40, 52], 31% for Major Depressive Episode and 20% for Generalized Anxiety
Disorder [36]. Individuals with poor mental health also report higher levels of disability and pain [14, 53].
Disorder [36]. Individuals with poor mental health also report higher levels of disability and pain [14, 53].
In order to improve recovery rates and decrease costs associated with WL injury, it is important that clinical recovery pathways, mechanisms underlying the initiation and maintenance of the condition, and prognostic or risk factors for poor recovery are understood. Without this knowledge, there is limited basis on which to develop more effective treatments to prevent the transition to chronic pain. This chapter will first outline the above factors before discussing the implications for the development and testing of new treatments.
Cohort studies have demonstrated that recovery, if it occurs, takes place within the first 2-3 months postinjury with a plateau of symptoms following this time point [34, 52]. Irrespective of long-term outcome, there is an initial decrease in symptoms to some extent in this early postinjury period. Two cohort studies (conducted in Denmark and Australia) have used trajectory-based modelling analyses to identify recovery pathways for disability, and both studies demonstrated similar results [2, 52]. The identified Australian pathways are illustrated in Fig. 8-1. These included: (1) a pathway of good recovery with initial mild to moderate levels of pain-related disability, and good recovery at 12 months with 45% of people predicted to follow this pathway; (2) a pathway of initial moderate to severe pain-related disability with some recovery but with disability levels remaining moderate at 12 months, with 39% of injured people predicted to follow this pathway; and (3) a pathway of initial severe pain-related disability and some recovery to moderate/severe disability, with 16% of individuals predicted to follow this pathway. The Australian study also explored mental health recovery pathways using the total symptom score of the Posttraumatic Stress Diagnostic Scale (PDS) [19]. Three very similar trajectories to disability were identified, and these are illustrated in Fig. 8-2—(1) Resilient: mild PTSD
symptoms throughout; (2) Recovering: initial moderate symptoms declining to mild levels by 3 months; and (3) Chronic moderate to severe: persistent moderate/severe symptoms throughout the 12-month study period. Further analysis revealed a synchrony between the disability and PTSD symptoms trajectory groups, suggesting a common link between chronic WAD and PTSD development following WL injury [52].
symptoms throughout; (2) Recovering: initial moderate symptoms declining to mild levels by 3 months; and (3) Chronic moderate to severe: persistent moderate/severe symptoms throughout the 12-month study period. Further analysis revealed a synchrony between the disability and PTSD symptoms trajectory groups, suggesting a common link between chronic WAD and PTSD development following WL injury [52].
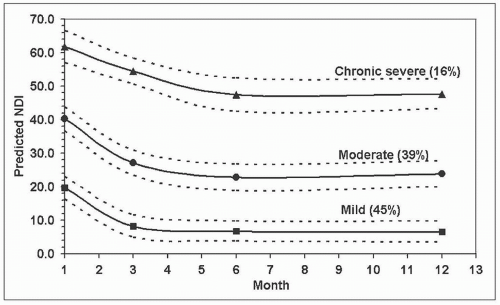 FIGURE 8-1 Predicted NDI trajectories with 95% confidence limits and predicted probability of membership (%). Figure reproduced from Sterling et al. [23], with permission International Association for the Study of Pain (IASP). |
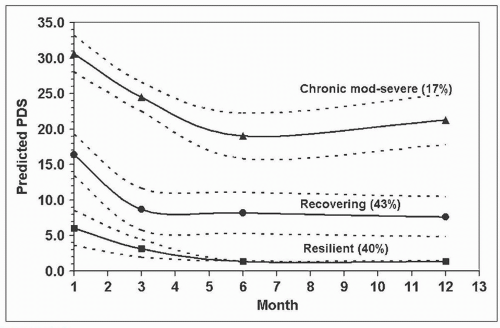 FIGURE 8-2 Predicted PDS trajectories with 95% confidence limits and predicted probability of membership (%). Figure reproduced from Sterling et al. [23], with permission International Association for the Study of Pain (IASP). |
The most consistent predictor of poor recovery following WL injury is the intensity of neck pain at the initial or baseline assessment, and this factor has been identified by all systematic reviews on this topic [10, 34, 46, 69, 70]. Walton et al. [69] synthesized the data from several cohort studies and established a cut-off point of 5.5 out of 10 on a visual analogue pain scale, with pain greater than this demonstrating a nearly sixfold increase in the risk of persistent pain or disability at follow-up. This factor was slightly more robust at predicting disability over pain outcomes [69]. Initial moderate-to-high levels of pain-related disability have also shown predictive capacity [57]. Interestingly, this is also the case for chronic pain development postsurgery and serious trauma. Higher levels of pain either before or in the acute postsurgery period are associated with an increased risk of developing chronic pain [1, 21, 24]. Initial pain severity has also been shown to predict chronic pain 12 months after serious orthopedic injury [27].
Mechanisms underlying higher levels of reported pain are not clear, but their elucidation may provide potential targets for treatment. Several studies have investigated the prognostic capacity of sensory measures with the aim of providing information on potential nociceptive processes that may underlie the development of chronic WL pain. Cold hyperalgesia predicted disability and mental health outcomes at 12 months postinjury [53, 55], and decreased cold pain tolerance measured with the cold pressor test predicted ongoing disability [35]. A recent systematic review concluded that there is now moderate evidence available to support cold hyperalgesia as an adverse prognostic indicator [22]. Other sensory measures such as lowered pressure pain thresholds (mechanical hyperalgesia) show inconsistent prognostic capacity. Walton et al. [68] showed that decreased pressure pain thresholds over a distal site in the leg predicted neck pain-related disability at 3 months postinjury, but other studies have shown that this factor is not an
independent predictor of later disability [55]. The exact mechanisms underlying the hyperalgesic responses are not clearly understood, but are generally acknowledged to reflect augmented nociceptive processing in the central nervous system (central sensitization or less of descending inhibition) [11, 58].
independent predictor of later disability [55]. The exact mechanisms underlying the hyperalgesic responses are not clearly understood, but are generally acknowledged to reflect augmented nociceptive processing in the central nervous system (central sensitization or less of descending inhibition) [11, 58].
Psychological factors that have shown the most consistent prognostic capacity for WAD outcomes include posttraumatic stress symptoms, pain catastrophizing, and symptoms of depressed mood [7, 54, 69]. Additionally, lower expectations of recovery have been shown to predict poor recovery where patients who do not expect to recover well may not do so [6, 26].
Most prognostic studies of WAD have been Phase 1 or exploratory studies with few confirmatory or validation studies yet conducted [50]. A recent study undertook validation of a set of prognostic indicators including initial disability, cold hyperalgesia, age, and posttraumatic stress symptoms. The results indicated that the predictive set showed good accuracy to discriminate patients with moderate/severe disability from patients with full recovery or residual milder symptoms at 12 months postinjury [54]. Such a validation study is rare in this area of research and goes some way toward providing greater confidence for the use of these measures in the early assessment of WL injury.
On the basis of results of previous cohort studies, a clinical prediction rule to identify both chronic moderate/severe disability and full recovery at 12 months postinjury was recently developed [44, 45]. The aim of the rule is to provide clinicians with a useful screening tool to gauge the risk of an individual patient developing a chronic condition. Initial disability levels (Neck Disability Index [NDI] score of ≥40%), age ≥35 years, and a score of ≥6 on the hyperarousal subscale of the PDS [20] could predict patients with moderate/severe disability at 12 months with good specificity, fair sensitivity, and a positive predictive value of 72% [45]. It is also important to predict patients who will recover well as these patients will likely require less intensive intervention. Initial NDI scores of ≤32% and age ≤35 years predicted full recovery at 12 months postinjury with a positive predictive value of 71% [45]. A third medium risk group could either recover or develop chronic pain and disability (>32% on the NDI, score >3 on
the hyperarousal subscale). The hyperarousal subscale comprises 5 items that evaluate the frequency of symptoms including: having trouble falling asleep, feelings of irritability, difficulty concentrating, being overly alert, and being easily startled. The clinical prediction rule has subsequently been externally validated in an independent cohort and the reproducibility and accuracy of this dual pathway tool have been confirmed for individuals with acute WAD [44]. It is illustrated in Fig. 8-3.
the hyperarousal subscale). The hyperarousal subscale comprises 5 items that evaluate the frequency of symptoms including: having trouble falling asleep, feelings of irritability, difficulty concentrating, being overly alert, and being easily startled. The clinical prediction rule has subsequently been externally validated in an independent cohort and the reproducibility and accuracy of this dual pathway tool have been confirmed for individuals with acute WAD [44]. It is illustrated in Fig. 8-3.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


