History and Development of Prosthetic Replacement of the Glenohumeral Joint
Wayne Z. Burkhead
Daryle Anthony Ruark
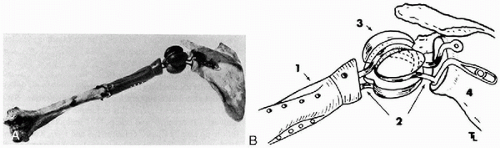
From the late 19th century until the early 1970s surgeons tried with varying degrees of success to develop a viable replacement for the human shoulder. The first successful mechanical replacement of the shoulder (Fig. 1-1) was performed in March 1882 by Jules E. Pean (1,2). Using a platinum tube and a paraffin-hardened rubber implant designed by the Parisian dentist J. Porter Michaels, Pean replaced the tuberculosis-infected shoulder of a 32-year-old Parisian (3). Although the implant failed because of underlying sepsis, because of Pean and Michael’s ingenuity the patient was spared a shoulder disarticulation and painful death. The principles of modern implant designs stem from their experience. In the following years numerous attempts were made to provide an aseptic, nonresorbable, well-tolerated device that preserved motion.
Prior to Pean’s report, several investigators had tried unsuccessfully to use ivory and xenograft tissue to replace human joints. Subsequent to Pean’s intervention, ivory was again used for shoulder reconstruction (4) in addition to a number of techniques with autograft tissues and bone (5, 6, 7, 8, 9, 10, 11, 12, 13, 14). This chapter will concentrate on the use of
mechanical devices to replace the glenohumeral joint and will trace their development until 1982.
mechanical devices to replace the glenohumeral joint and will trace their development until 1982.
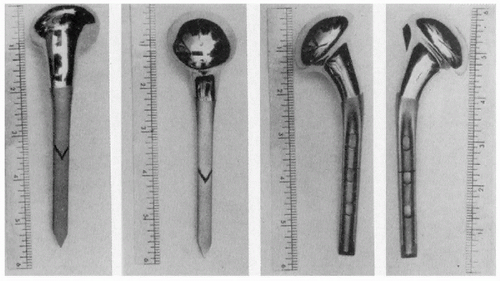
An acrylic prosthesis (Fig. 1-2) was first used by Baron and Senn to replace the proximal humerus in 1951 (15). Beginning in 1953, de Anquin (16) performed 11 acrylic shoulder replacements for fracture and had one of the first designs allowing tendinous reattachment around the prosthesis. Although the poor wear characteristics of acrylic, anchorage difficulty, and component breakage sealed the fate of these implants, their use continued until as late as 1969 (17).
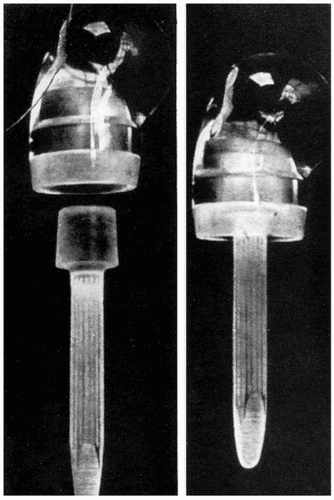 Figure 1-2 An acrylic prosthesis designed to replace the proximal humerus after fractures and fracture dislocations. (From Richard et al, with permission.) |
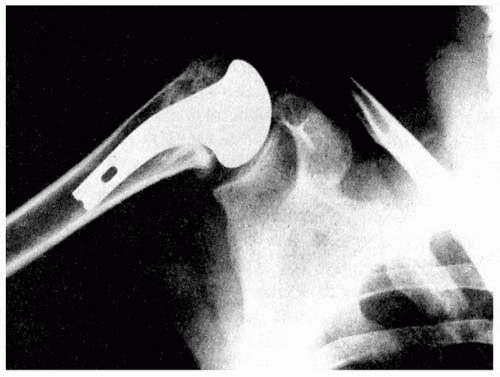
The first modern shoulder arthroplasty was heralded by Frederick Krueger on December 12, 1950 (18). An anatomically shaped, bioinert device was created by Austinol Laboratories in New York. Based on acrylic molds of cadaveric shoulders, a vitallium mold prosthesis (Fig. 1-3) was created and implanted into the shoulder of a merchant marine who had developed avascular necrosis. A well-functioning and painless shoulder was obtained following the cementless implantation of this device with its fenestrated stem for bone ingrowth (Fig. 1-4). Krueger’s surgical technique involved excising the head down to the capsule while carefully preserving the rotator cuff tendon attachments. This conservative rotator cuff-sparing method remains one of the most important concepts of shoulder replacement surgery.
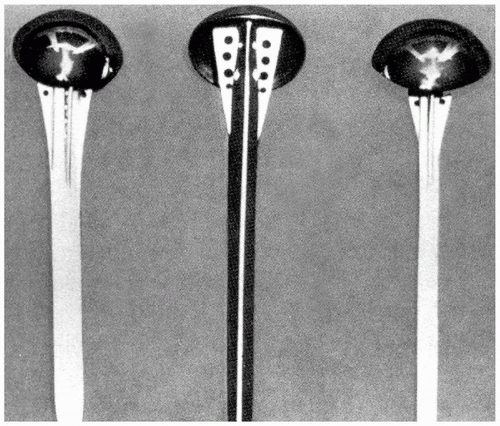
Charles S. Neer II was stimulated to work on shoulder replacements by patients who had persistent pain, limited motion, and poor function following simple head excision for complex proximal humeral fractures (19). In 1953 he designed a prosthesis that he postulated would serve as a
stable fulcrum for important shoulder force couples (Fig. 1-5). The implant subsequently was modified to facilitate tuberosity fixation and allow for bony ingrowth and stability (Fig. 1-6). Neer reported a series of 12 noncemented Neer I arthroplasties with follow-up ranging from 2 to 23 months and revealed that 11 of the 12 patients were pain free (20). The functional results were excellent or satisfactory in the majority of patients. Poor results were seen in only two patients whose surgery had been delayed for several months. In addition to the prosthetic design, two key concepts also emerged. Neer stressed the importance of tuberosity fixation and healing and the benefits of early surgical intervention.
stable fulcrum for important shoulder force couples (Fig. 1-5). The implant subsequently was modified to facilitate tuberosity fixation and allow for bony ingrowth and stability (Fig. 1-6). Neer reported a series of 12 noncemented Neer I arthroplasties with follow-up ranging from 2 to 23 months and revealed that 11 of the 12 patients were pain free (20). The functional results were excellent or satisfactory in the majority of patients. Poor results were seen in only two patients whose surgery had been delayed for several months. In addition to the prosthetic design, two key concepts also emerged. Neer stressed the importance of tuberosity fixation and healing and the benefits of early surgical intervention.
By 1956 de Anquin (16) had modified his original acrylic stem to a metallic component. The noncemented, fenestrated prosthesis was similar to that of Krueger and Austin Moore. A subsequent design modification using a polyethylene head failed rapidly and was abandoned.
Neer continued to apply his techniques with only minor alterations. His goal of providing a sliding fulcrum while also providing leverage was realized by creating a loose fit in the glenoid, performing accurate subscapularis repair, and paying attention to detail in regard to proper version of the prosthesis (21). He reported on 48 replacement arthroplasties for glenohumeral arthritis in 1974 (22). All cases were noncemented hemiarthroplasties except for one total shoulder replacement with an all-polyethylene glenoid component. Neer described the structural alteration of primary glenohumeral arthritis, including thinning of the articular cartilage, as well as eburnated and sclerotic bone, most advanced at the area of maximum humeral contact and joint reaction force. Degenerative subarticular cysts were noted just superior to the midpoint of the articular surface. The largest osteophytes were located at the inferior margin of the joint. The articular surface of the glenoid was smooth but usually consisted of eburnated bone devoid of cartilage. Marginal osteophytes could be palpated in the ligaments of the glenoid. In addition, Neer also described the technique of subscapularis Z-plasty lengthening and recommended increasing humeral retroversion up to 35 degrees.
The results of this series of patients showed 20 patients with excellent results, 20 with satisfactory results, and 6 with unsatisfactory results (22). The follow-up ranged from 1 to 20 years with an average of 6 years. He reported no evidence of stem loosening or any glenoid flattening, sclerosis, or enlargement when compared to preoperative radiographs. A postmortem specimen showed permeation and filling of the holes in the proximal portion of the prosthesis. He concluded that a properly performed hemiarthroplasty combined with release of contractures could be expected to slow the deterioration of the glenohumeral joint, relieve pain, and allow normal use.
Even with these successful results, Neer continued to develop new ideas. Between 1970 and 1974, with the assistance of Robert Averill, he developed and later modified a fixed fulcrum shoulder replacement (23). Their first design was a conventional ball-and-socket joint. The second prosthesis was a reverse ball-and-socket design. Finally, the Mark III (Fig. 1-7) was also a reverse ball-and-socket joint but with a unique dual-compartment humeral component allowing axial rotation of the metal stem within a polyethylene sleeve. However, the devices failed despite these ingenious attempts to reduce stresses at bone interfaces as well as component articulations. Based on the failure of his constrained prostheses and many others developed in Europe and the United States, Neer abandoned constrained arthroplasty, predicting that leverage would be transferred to the fixation of the glenoid component with loosening or fracture occurring in the active patient. By 1972 his guiding principles were well-established (i.e., minimal bone resection, anatomic designs, avoidance of mechanical impingement, and repair and rehabilitation of the soft tissues).
 Figure 1-7 The Neer Mark III fixed-fulcrum prosthesis. Note the reverse ball-and-socket configuration and rotating metallic stem. (Reproduced with permission from Neer.) |
Although Neer warned of impending failures, many investigators still believed that the problems of rotator cuff and capsular-deficient shoulders would be best addressed with constrained devices. Some felt that the problems related to prosthesis loosening could be addressed with the use of methylmethacrylate or low-friction devices as pioneered by Sir John Charnley with hip arthroplasties. The Bickel shoulder prosthesis, designed at the Mayo Clinic (24), incorporated these concepts with the use of both cement and a small metallic ball on the humeral stem articulating with a larger-radius polyethylene socket. The operation was described as difficult, and the reoperation rate was 50% at 5 years as a result of early glenoid loosening and fractures of the prosthesis and the glenoid.
Other investigators directed their ingenuity to optimizing glenoid component fixation. Lettin and Scales (25) designed the Stanmore shoulder prosthesis that used three extra-long pegs cemented into the scapula. They later modified the design to displace the instant center of rotation, attempting to increase range of motion and decrease stresses on the component interface (26). In a 1982 report, range of motion was inconsistent and disappointing and 10 of 50 replacements required revision surgery as a result of glenoid loosening (27).
As secure glenoid fixation was attained in constrained prostheses, other complications developed. In 1979 Post (28) introduced a cemented glenoid component with screw fixation. Because glenoid fixation was significantly increased, the possibility of scapula fractures was the major concern. To avoid this complication, the constrained prosthesis was designed to dislocate at a force lower than that required to fracture the scapula. In the initial series, 11 of 22 patients had revision surgery for dislocations, humeral component plastic deformation, and humeral component fracture. Even after the humeral component was changed from stainless steel to cobalt chrome alloy, subsequent reports revealed persistently high revision rates (29, 30, 31).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree