Stroke is a significant source of mortality and long-term disability in the United States. Of persons who survive a stroke, approximately 50% will have hemiplegia, half of whom will live with a nonfunctional arm. Hemiplegic shoulder pain (HSP), which occurs in most patients with hemiplegia, reduces participation and worsens outcomes in rehabilitation. Management of HSP is challenging because its causes are multifactorial and there is limited, conflicting, or nonspecific evidence in support of most treatments. This article develops an effective approach for diagnosis and treatment using the best available evidence to aid practitioners in obtaining optimal results.
Key points
- •
Hemiplegic shoulder pain (HSP) occurs in most patients with hemiplegia, and has an adverse effect on functional outcomes.
- •
Evaluation and management is challenging, as HSP remains a clinical diagnosis, and many of the available treatments for HSP lack sufficient or robust support in the medical literature.
- •
The pathogenesis of HSP is multifactorial and includes neurologic and mechanical factors, often in combination, which vary among those affected.
- •
The systematic approach discussed in this article is intended help practitioners to accurately identify the factors contributing to each patient’s pain, and to prescribe the most effective treatment based on the available evidence.
Introduction
Stroke, or cerebrovascular accident, is the third leading cause of death and the leading cause of adult long-term disability in the United States. Impairments from stroke vary widely, but one of the most common is hemiplegic shoulder pain (HSP). Pain and loss of function in the upper limb is a significant detriment to quality of life. HSP is a challenge to patients and their health care providers, as it reduces participation in rehabilitation, discourages motion, hinders recovery, and adversely affects function. The causes of HSP are multifactorial, have neurologic and mechanical causes, and can be generated peripherally in the limb or centrally within the brain.
Although HSP has been recognized and discussed in the medical community for decades, the evidence in the medical literature lacks sufficient quantity and quality, and is inconsistent in its conclusions. It can be confusing to manage HSP when each of its components has its own controversies in treatment. For example, even if adhesive capsulitis is identified as a contributor to HSP, debate remains regarding the best treatment practice for adhesive capsulitis itself. The purpose of this article is to assist the reader in developing a strategy for the management of HSP. No patient is exactly the same, so a one-size-fits-all treatment is unlikely to be effective. Instead, the focus should be on a consistent approach to ensure that all components of the diagnosis are addressed appropriately.
Introduction
Stroke, or cerebrovascular accident, is the third leading cause of death and the leading cause of adult long-term disability in the United States. Impairments from stroke vary widely, but one of the most common is hemiplegic shoulder pain (HSP). Pain and loss of function in the upper limb is a significant detriment to quality of life. HSP is a challenge to patients and their health care providers, as it reduces participation in rehabilitation, discourages motion, hinders recovery, and adversely affects function. The causes of HSP are multifactorial, have neurologic and mechanical causes, and can be generated peripherally in the limb or centrally within the brain.
Although HSP has been recognized and discussed in the medical community for decades, the evidence in the medical literature lacks sufficient quantity and quality, and is inconsistent in its conclusions. It can be confusing to manage HSP when each of its components has its own controversies in treatment. For example, even if adhesive capsulitis is identified as a contributor to HSP, debate remains regarding the best treatment practice for adhesive capsulitis itself. The purpose of this article is to assist the reader in developing a strategy for the management of HSP. No patient is exactly the same, so a one-size-fits-all treatment is unlikely to be effective. Instead, the focus should be on a consistent approach to ensure that all components of the diagnosis are addressed appropriately.
Scope and significance
Every year in the United States 795,000 people suffer a new or recurrent stroke: 1 stroke every 40 seconds. More than 7 million Americans older than 20 years have had a stroke. Stroke is the third leading cause of death and the leading cause of long-term disability, costing the United States $18.8 billion annually, and with a lifetime cost of $140,000 per patient with ischemic stroke. Of those who survive a stroke, approximately half have hemiplegia. Although 70% of those with hemiplegia will achieve ambulatory status, half are left with a nonfunctional arm. The incidence of HSP is widely reported in previous literature, ranging from 16% to 84% but most commonly reported as near 70%.
It is not only pain but associated psychological distress that limits a patient’s participation in the rehabilitation process. The presence of HSP is strongly correlated with a prolonged hospital stay and lower Barthel functional score in the first 12 weeks after stroke. Of patients who had a Barthel Index score of less than 15, 59% experienced shoulder pain during their hospital stay, compared with 25% of patients with a Barthel Index score greater than 15. Patients with HSP are less likely to return to their home. Conversely, improvement of upper limb function within the first 5 weeks after a stroke can result in improved use of the affected limb in functional tasks.
Predictors and prognosis in hemiplegic shoulder pain
HSP has a significant impact on function both during and after rehabilitation. A meta-analysis of 58 studies assessed outcomes of overall upper limb recovery according to age, sex, lesion site, initial motor impairment, motor-evoked potentials, and somatosensory-evoked potentials. Only initial measures of impairment and function predicted long-term outcome. Age in itself is not clearly a risk factor on its own, but those of older age are more likely to have preexisting abnormality that affects impairment. Additional risk factors for developing shoulder pain within the first 6 months after stroke include impaired voluntary motor control, diminished proprioception, tactile extinction, abnormal sensation, spasticity of the elbow flexor muscles, restricted range of motion (ROM) for both shoulder abduction and shoulder external rotation trophic changes, and type 2 diabetes mellitus. Barlak and colleagues found a significant correlation between HSP and adhesive capsulitis and complex regional pain syndrome, but none between HSP and grade of subluxation, spasticity, impingement syndrome, or thalamic pain.
In addition to new impairments following a stroke, the practitioner must also consider the likelihood of pre-existing abnormality, whether symptomatic or not, which may contribute to pain in the shoulder. Shoulder pain is a common musculoskeletal complaint made to primary care physicians and a reason for referral to a musculoskeletal specialist. Rotator cuff disorders are the most common source of such pain. Partial tears of the rotator cuff are frequently seen as early as age 50 years, with the risk of severe injury increasing in the 60s and 70s age groups. Degeneration of articular surfaces may reduce ROM, and damage to soft tissue can increase joint laxity. Further complicating a proper diagnosis are data that suggest a poor correlation between symptoms and findings on physical examination. Dromerick and colleagues found that examination findings consistent with injury to the supraspinatus and long head of the biceps are more consistently associated with early onset of HSP, regardless of whether the patient reported pain.
Shoulder anatomy
The human shoulder is a complex ball-and-socket joint that allows multidirectional reach. This agility comes at the sacrifice of stability. The extensive ROM is due largely to the shallow depth of the glenoid fossa, with only 25% of the humeral head coming into contact with the glenoid. This agility is necessary to properly position the hands for a large variety of functional tasks. The only true joint directly connecting the entire upper quarter to the trunk is the sternoclavicular joint. Stability of movement, therefore, depends on both static and dynamic stabilizers ( Fig. 1 ). Stability is provided by the surrounding muscles and ligaments. The glenohumeral ligaments serve as the primary static stabilizers and include the superior, middle, and inferior glenohumeral ligaments. The primary dynamic stabilizers are the rotator cuff muscles, whose attachments form a cuff around the head of the humerus.
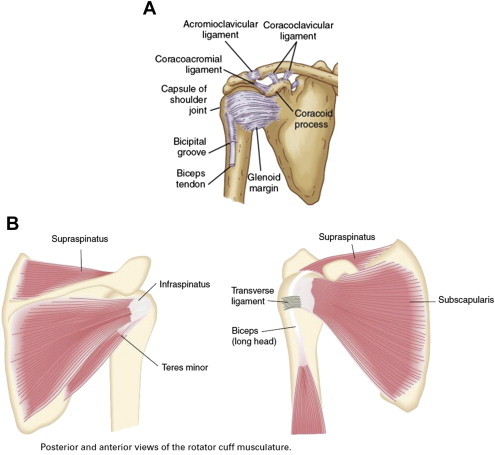
The glenohumeral joint derives passive support from a cartilaginous labrum, glenohumeral ligaments, and joint capsule. Functional movements require coordinated movements of dynamic stabilizers. The deltoid and rotator cuff muscles (supraspinatus, infraspinatus, teres minor, and subscapularis) act on the humerus, and the position of the scapula is primarily controlled by the trapezius, serratus, and latissimus dorsi. The subscapularis rotates the humerus internally, whereas the infraspinatus and teres minor are external rotators. Abduction is primarily achieved by the deltoid and is aided by the supraspinatus. The rotator cuff muscles compress the humeral head in the glenoid fossa, thereby stabilizing the joint and providing a counterbalance to opposing forces on the humerus. Overhead activity requires simultaneous abduction by the deltoid and external rotation by the infraspinatus. Movements in a single anatomic plane, such as abduction, can only be accomplished with a predictable ratio of movement termed scapulohumeral rhythm. Impairment of rotator cuff action can lead to superior subluxation of the humeral head, predisposing to impingement of the supraspinatus between the greater tubercle of the humerus and the acromion.
Mechanisms of injury
Although many mechanisms for HSP have been proposed, pinpointing the cause in individual patients can be elusive. The etiology may be multifactorial, relating to disruption of the biomechanical balance of the shoulder caused by stroke-induced weakness, spasticity, and sensory impairment. Several systems for categorizing HSP exist. A model by Ryerson and Levit identified 4 major sources of pain in patients with HSP. Joint pain resulting from instability can cause sharp pain with passive or active movement. Atrophic or spastic muscle can result in a “pulling” pain with movement. Abnormal pain sensitivity can arise from inappropriate central nervous system modulation of the pain, which can vary from diffuse and achy to sharp and lancinating. Complex regional pain syndrome, though less common, is characterized by reduced ROM, dysesthesia, and trophic changes.
The difficulty in interpreting this and other descriptions of HSP is the absence of any pathognomonic relationship to any particular subtype of pain. Achy pain emanating from muscle or tendon impingement can just as likely result from an upper motor neuron disorder such as spasticity. Sharp pain, allodynia, or hyperpathia caused by a lower motor neuron disorder, such as axillary neuropathy, could present with similar symptoms associated with central causes of pain and altered sensation. To avoid such confusion, the classification of HSP is more accurately based on etiology rather than symptoms alone.
Approach to differential diagnosis
To more effectively determine the factors that contribute to hemiplegic shoulder pain, the authors suggest that factors affecting HSP should be divided into 2 categories: neurologic and mechanical ( Box 1 ). Neurologic factors include spasticity, brachial plexus injury, complex regional pain syndrome (CRPS), and central sensitization. Mechanical factors include shoulder subluxation, rotator cuff injury, glenohumeral joint disorders, adhesive capsulitis, and direct trauma. It is important to appreciate that the cause of pain may involve a combination of neurologic and mechanical factors.
Neurologic Factors
Upper motor neuron neurologic factors
Paralysis, spasticity, central poststroke pain, central sensitization
Lower motor neuron neurologic factors
Peripheral neuropathy, brachial plexus injury, complex regional pain syndrome
Mechanical Factors
Shoulder subluxation, rotator cuff injury, glenohumeral joint disorders, adhesive capsulitis, myofascial pain, direct trauma
Neurologic Factors
Weakness
Weakness of the muscles supporting the shoulder joint is a commonly seen after a stroke and often persists chronically. Weakness disrupts the stabilizers of the shoulder joint and often precedes subsequent development of spasticity. It is an underlying factor common to both neurologic and mechanical factors. Weakness of the trunk muscles and the muscles stabilizing the head is also common after stroke and frequently affects posture, most commonly creating a forward flexed and stooped posture, which can further lead to anterior subluxation of the shoulder and further exacerbate rotator cuff impingement and traction on the joint capsule.
Spasticity
Muscle spasticity is commonly defined as a velocity-dependent resistance to passive stretch. It is a consequence of an upper motor neuron disorder, creating an imbalance between agonist-antagonist muscle pairs. The result in hemiplegia is typical posturing with a dominant flexor tone in the upper limbs. Overactivity of the pectoralis and subscapularis is most predominant about the shoulder, resulting in excessive humeral flexion, adduction, and internal rotation. Combined with increased activity of teres major and latissimus dorsi, spasticity inhibits active and passive abduction, extension, and external rotation at the shoulder. The consequence is inability to achieve desired ROM for activities of daily living (ADLs), and predisposition to mechanical injury (eg, rotator cuff impingement).
Of patients with HSP, approximately 85% with spastic hemiplegia experienced pain, compared with 18% in those with a flaccid hemiplegia. Patients with reduced external rotation experience more pain, and use of a subscapular nerve block to a spastic subscapularis muscle has been demonstrated to reduce pain. Preservation of joint mobility in patients with spasticity and prevention of contracture in those with flaccid hemiplegia are intended to reduce the incidence of HSP.
Brachial plexus and peripheral nerve injury
The brachial plexus is derived from C5-T1 roots, and arises at the lower aspect of the neck. It runs behind scalenes proximally, and behind the clavicle and pectoralis muscles distally. Injury to the plexus can be traumatic or atraumatic. In the setting of hemiplegia, the cause is most likely a traction injury caused by improper handling of the flaccid hemiplegic limb, such as pulling on the arm during transfers and repositioning. One study based on needle electromyography (EMG) reported that 75% of supraspinatus and deltoid muscles in hemiplegic arms had neuropathic responses. The upper trunk of the plexus is most susceptible to injury. The most common isolated peripheral nerve injury in HSP is axillary neuropathy, thought to be subsequent to downward displacement of the humeral head in shoulder subluxation. However, other studies have failed to reveal significant evidence of plexus or peripheral nerve injuries associated with HSP. Given the conflicting evidence, it is not possible to ascertain whether plexopathy or mononeuropathy plays a substantial role in HSP. However, if a plexus or peripheral nerve injury occurs it may contribute to a cycle of pain, weakness, and progressive subluxation.
Complex regional pain syndrome
Type 1 CRPS, previously termed reflex sympathetic dystrophy or shoulder-hand syndrome, and Type 2 CRPS, previously termed causalgia, are characterized by pain that is out of proportion to the pathologic condition, peripheral and/or central autonomic abnormalities, and dystrophic changes to a limb often (but not always) following a traumatic injury. CRPS can inhibit mobility by both pain that discourages motion and the associated adhesive capsulitis that restricts it. The incidence of CRPS in patients with hemiplegia has been cited to be as high as 23%. However, there is considerable variability in past reported incidence, likely attributable to various diagnostic criteria. The precise mechanism of this disorder remains unclear. There are studies demonstrating an association between shoulder-hand syndrome and spasticity, confusion, and sensory loss. Damage to the soft tissues surrounding the hemiplegic shoulder have been implicated as a cause of shoulder-hand syndrome. Abnormalities in the brain itself have also been implicated. Further study is needed before a definite causality between HSP and CRPS can be confirmed.
Central poststroke pain and sensitization
Sensory disturbance and neglect can alter a patient’s proprioception and perception of pain, predisposing the shoulder to injury. Central poststroke pain (CPSP) is another impairment deriving from stroke that can contribute to pain in the shoulder and elsewhere. Also termed thalamic pain syndrome, a lesion of the spinothalamocortical pathway may result in abnormal neural reorganization. The result is an improper generation of pain in the absence of injury, which can be reported as neuropathic, spastic, or musculoskeletal in quality. Central sensitization is a separate entity that can be observed in the presence of CRPS and CPSP, whereby abnormal responsiveness of nociceptive neurons results in dysesthesia. Sensitization often involves alterations in neurotransmitter levels, including serotonin and norepinephrine.
Mechanical Factors
Shoulder subluxation
Shoulder subluxation refers to the static displacement of the humeral head in relation to the glenoid, and represents a common source of mechanical pain in HSP. Subluxation requires a disruption in the integrity of the glenohumeral joint. Clinical findings are a gap between the humeral head and the acromion. This gap can be measured with calipers, radiography, or ultrasonography, but is commonly described by finger breadths in the clinical setting. During the early stages following stroke the muscles in the hemiplegic arm are usually flaccid, thereby impairing joint stability and predisposing the shoulder to traction-type injury. The most common reason is an inability of the paralyzed shoulder girdle musculature to provide dynamic stability at the joint. Articular tissues (eg, the joint capsule) can become distended, particularly in the flaccid stage following stroke. This distension is also hypothesized to contribute to ischemia in the tendons of the supraspinatus and long head of the biceps. Downward displacement of the humerus is most common during the flaccid stage, whereas the spastic stage often leads to anterior displacement, posterior displacement, or internal rotation. Anteroposterior (AP) and oblique radiographs help diagnose and characterize shoulder subluxation. Clinical diagnosis of subluxation is often achieved by measuring arm-length discrepancy or by palpating or measuring the subacromial space.
The association between shoulder subluxation and HSP remains controversial. Paci and colleagues studied 107 patients with hemiplegia in a case-control design, and measured the presence of shoulder pain in those with shoulder subluxation and those without. Patients with shoulder subluxation had significantly greater pain at admission, discharge, and at a 30- to 40-day follow-up assessment; they also had greater impairment with ADLs and required longer hospital stays. However, other studies argue that patients without subluxation are just as likely to develop pain. Comparative studies of the association between shoulder subluxation and pain are limited by sample size or methodology. However, there is enough evidence to suggest that shoulder subluxation may be a contributing factor in HSP. Proper positioning, support, and correct transfer techniques by caregivers may be helpful in prevention and alleviation of pain.
Rotator cuff injury
As discussed previously, the primary purpose of the rotator cuff is to stabilize the humeral head relative to the glenoid during shoulder movements. Rotator cuff injuries are a common source of shoulder pain in the general population. Rotator cuff tears occur in 20% to 40% of the general population, with increasing incidence with age. The incidence of rotator cuff tears in hemiplegic patients ranges from 33% to 40%. It is unlikely that hemiplegia is a cause of rotator cuff injury per se, but abnormal positioning, muscle imbalance caused by weakness, and spasticity can all increase the likelihood of impingement and tearing. In addition, falls can be a common occurrence during the initial onset of the stroke itself, and may be a cause of rotator cuff tear, which may go unnoticed during the initial stages of the stroke. Improper handling of the hemiplegic arm could also cause injury to the rotator cuff tendons. Treatment of rotator cuff injuries in the plegic or paretic arm is usually conservative and supportive.
Adhesive capsulitis
The term frozen shoulder is often used to describe a shoulder with decreased ROM, but the term is nonspecific and fails to determine how much of the restriction is passive (ie, a block to motion) versus active (ie, limited by pain or weakness). Adhesive capsulitis is a more specific term that refers to a condition of uncertain origin characterized by significant restriction of both active and passive shoulder motion that occurs in the absence of a known intrinsic shoulder disorder. A painful shoulder may develop adhesive capsulitis because of pain inhibition of mobility, leading to subsequent disuse atrophy and contracture. The pain of adhesive capsulitis is also theorized to lead to increased immobility. The decreased ROM can lead to inflammation, muscle atrophy, and contracture resulting from adhesions. The prognosis of adhesive capsulitis is favorable, but requires diligence to preserve available ROM and strength. Increased immobilization from spasticity can increase the likelihood of developing adhesions.
Myofascial pain
A more complete discussion of myofascial pain and trigger-point theory can be found in other articles by Dr Gerwin and by Dr Borg-Stein and Iaccarino elsewhere in this issue. As with most musculoskeletal disorders, it is important for the clinician to consider the contribution of muscle-generated pain from muscles about the shoulder girdle, and the contribution to posture and muscle balance on the level of myofascial pain. Although there is a larger body of data regarding the impact of myofascial pain on shoulder pain in the general population, there is only one published study specifically studying myofascial pain in HSP, which demonstrated improvement of pain with dry needling of trigger points when combined with standard rehabilitation.
Diagnosis of hemiplegic shoulder pain
There are no clear or widely accepted criteria for diagnosing HSP. Therefore, the authors recommend confirming the diagnosis following the same approach of dividing the workup according to suspected neurologic and mechanical factors ( Box 2 ).
History, physical examination, special tests/maneuvers
Imaging (radiography, magnetic resonance imaging, ultrasonography)
Electrodiagnosis
Diagnostic nerve blocks or injections (intramuscular, intra-articular)
Neurologic Factors
History and physical examination
A proper history and physical examination are paramount, especially when symptoms can be explained by multiple causes. Important information to elicit during history taking includes preexisting shoulder pain and use of analgesics, limited functional use of the arm, prior trauma, and surgery. Regardless of the diagnosis, the key steps in the physical examination include observation (for asymmetry, deformity, and erythema), ROM, palpation, sensation, reflexes, strength, and special tests. The patient should demonstrate maximum active range of motion (AROM) before the examiner assesses full passive range of motion (PROM). Pain is most often the limiting factor in AROM, followed by weakness. If there is reduced PROM, contracture or anatomic block should be suspected. A goniometer can provide more objective monitoring of changes to ROM. Palpation is performed to assess for muscle bulk, abnormal contour or masses, or areas of tenderness. Key targets of palpation should include rotator cuff, deltoid, periscapular muscles, long head biceps tendon, other upper quarter musculature, and acromioclavicular joint. Strength testing in the C5-T1 myotomes (graded 0–5), sensory testing in the C5-T1 dermatomes (graded 0–2+), and C5-C7 reflexes (graded 0–4+) will help to localize a neurologic lesion, whether central or peripheral.
As with any neurologic injury, careful consideration to sensation and strength are useful in determining whether the lesion is central (brain and spinal cord) or peripheral, and whether the damage is focal (as in axillary neuropathy) or diffuse (as in CRPS). Because hemiplegia is an upper motor neuron disorder, it is also important to assess the presence and severity of spasticity. Muscle spasticity is determined using the Modified Ashworth Scale ( Box 3 ).
0: No increase in muscle tone
1: Slight increase in muscle tone, manifested by catch and release, or minimal resistance to the end range of motion (flexion or extension)
1+: Slight increase in muscle tone, manifested by catch and then minimal release through the remainder (less than half) of the range of motion
2: Moderate (marked) increase in muscle tone through most of the range of motion, but affected part is easily moved
3: Severe (considerable) increase in muscle tone through most of the range of motion, and affected part is difficult to move
4: Affected part is rigid in flexion or extension, little to no passive range of motion
Electrodiagnosis
Electrodiagnostic testing has excellent sensitivity and specificity for nerve injury within the peripheral nervous system. Electrodiagnostic testing may have limited utility in patients with HSP. Although it may be helpful in diagnosing a peripheral neuropathy, it cannot reliably exclude shoulder pain related to centrally mediated weakness or spasticity. Nevertheless, it may be useful in situations where there is underlying or concomitant possibility of brachial plexus nerve injury, peripheral mononeuropathy, or cervical radiculopathy.
Sympathetic block
A sympathetic ganglion block is a diagnostic option considered for patients with suspected CRPS. This block may assist in reducing symptoms mediated by the sympathetic nervous system, which includes alterations in skin color and temperature. These blocks will often cause a temporary Horner syndrome.
Mechanical Factors
Physical examination
The basic components of the physical examination, such as testing of strength, sensation, and reflexes, are used regardless of the cause of HSP. In addition, there are multiple specialized tests for the shoulder, but only a few most pertinent to the mechanical components of HSP ( Fig. 2 A–F ).
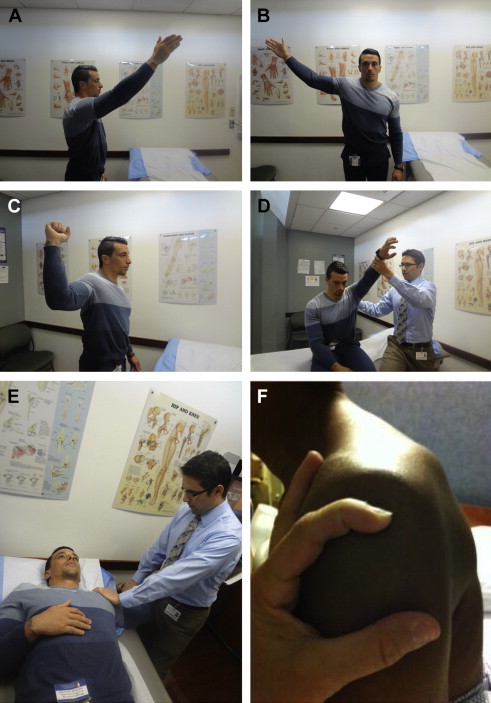
Neer, Hawkins, and Jobe (“empty can”) tests can assess for subacromial impingement. Apprehension and sulcus tests assess for glenohumeral joint instability. The apprehension test is performed by placing the patient in a supine position near the edge of the bed with the arm externally rotated, abducted, and in slight extension. Apprehension against further motion during the maneuver suggests anterior shoulder instability with 63% sensitivity. The sulcus test is performed in the sitting position with the affected arm at the patient’s side. The examiner pulls the elbow inferiorly to measure the physiologic separation between the acromion and humeral head. Separation of 1 cm is scored as Grade 1, 1 to 2 cm is scored as Grade 2, and more than 2 cm is scored as Grade 3. Grade 3 separation indicates multidirectional glenohumeral instability, but the maneuver has only 28% sensitivity.
The Neer test is performed by passive forward elevation of the arm with scapula stabilized. A modification of the test includes adding internal rotation of the humerus to approximate the acromion and greater tuberosity of the humerus. Positive pain using this maneuver suggests subacromial impingement with 88% sensitivity. The Hawkins test for impingement is positive if pain is produced with passive horizontal adduction and internal rotation. The Jobe (or empty can) test is positive for impingement if pain is produced when resistance is applied to arms elevated and internally rotated in the scapular plane (horizontal abduction to approximately 45°).
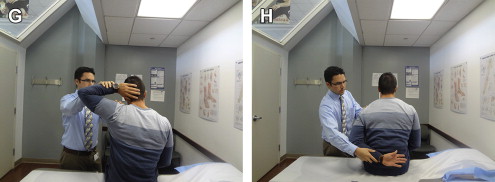
In stroke patients able to comply, a simple and efficient bedside screen for shoulder ROM includes the hand-behind-back (HBB) and hand-behind-neck (HBN) maneuvers. The HBB maneuver combines internal rotation and extension, and the HBN maneuver combines external rotation and abduction (see Fig. 2 G, H). Differences or pain in passive or active external rotation of the shoulder can indicate the onset of HSP.
The value of the physical examination is often greatest when multiple maneuvers are positive, or when overall movement is asymmetric relative to the unaffected side. Rajaratnam and colleagues concluded that HSP could be successfully diagnosed clinically by using only 3 of the aforementioned methods: Neer test, HBN, and a difference of greater than 10° passive external rotation at the shoulder joint. When combined with a report of at least moderate pain at rest, the sensitivity and positive predictive value for HSP was 96.7%. Another study of proprioception and kinematics of shoulder motion in patients with and without HSP found that those with HSP demonstrated increased lateral scapular rotation and decreased perception of passive movement. Furthermore, patients who had a stroke were more likely to demonstrate abnormal scapular movement on the nonparetic side when compared with controls, arguing that rehabilitation must always take both sides of the body into account.
Diagnostic imaging
Ultimately the diagnosis of HSP is clinical, and does not necessitate diagnostic imaging. However, the use of imaging may be of benefit if the history and examination raise suspicion of underlying traumatic or structural abnormalities that may contribute to the patient’s pain.
Radiography
Radiographic imaging is a useful starting point for evaluating suspected mechanical components of HSP. An AP view will rule out fracture and help to assess for subluxation. Adding AP views with the humerus in external rotation will bring the greater tuberosity and associated soft tissue into better view, and may help reveal calcific rotator cuff tendinopathy. Rotator cuff impingement by the acromion is best evaluated with a scapular Y view. If there is concern for shoulder instability, an axillary view will evaluate the relationship of the humerus to glenoid, and an AP view with humerus in internal rotation may reveal a Hill-Sachs lesion seen in traumatic dislocation.
Conventional and Magnetic Resonance Arthrography
Conventional x-ray arthrography is rarely used in clinical practice as an isolated method of diagnosis, but can help diagnose both adhesive capsulitis and rotator cuff tears. A normal joint will have a volume exceeding 10 mL, smooth glenohumeral capsular margin contours, and the presence of an axillary recess (a pouch of the capsule bordered by the inferior rim of the glenoid cavity and inferior portion of the humeral head). Patients with the presence of adhesive capsulitis demonstrate less than 10 mL of volume, irregular capsule margins, and a diminished or absent axillary recess. A rotator cuff injury will be demonstrated by contrast leakage from the glenohumeral joint to the subdeltoid bursa. The high sensitivity of arthrography (as high as 99%) makes this procedure the gold standard for detecting such tears. However, soft tissues cannot be visualized using this method.
A 1-year study of 32 patients with HSP by Lo and colleagues attempted to correlate arthrographic and clinical findings of HSP. Clinical measurements included Brunnstrom stage ( Box 4 ), spasticity distribution, presence or absence of shoulder subluxation, or CRPS Type 1. Arthrographic measurements included shoulder joint volume and capsular morphology. Fifty percent of the patients had evidence of adhesive capsulitis, 44% had shoulder subluxation, 22% had rotator cuff tears, and 16% had CRPS Type 1. Disorders were often present in combination. The study determined that arthrography was useful in identifying adhesive capsulitis, and that most cases developed within 2 months of developing HSP. Most significantly, outcomes worsened the longer adhesive capsulitis remained untreated. Even when diagnostic imaging is not used, the findings emphasize the importance of initiating appropriate treatment whenever adhesive capsulitis is clinically suspected.








