Abstract
Hemi-spatial neglect syndrome is common and sometimes long-lasting. It is characterized by a deficit in the use and awareness of one side of space, most often consecutive to a right hemisphere injury, mainly in the parietal region. Acknowledging the different types and all clinical characteristics is essential for an appropriate evaluation and adapted rehabilitation care management, especially as it constitutes a predictive factor of a poor functional prognosis. Some new approaches have been developed in the last fifteen years in the field of hemi-spatial neglect rehabilitation, where non-invasive brain stimulation (TMS and tDCS) holds an important place. Today’s approaches of unilateral spatial neglect modulation via non-invasive brain stimulation are essentially based on the concept of inter-hemispheric inhibition, suggesting an over-activation of the contralesional hemisphere due to a decrease of the inhibiting influences of the injured hemisphere. Several approaches may then be used: stimulation of the injured right hemisphere, inhibition of the hyperactive left hemisphere, or a combination of both. Results are promising, but the following complementary aspects must be refined before a more systematic application: optimal stimulation protocol, individual management according to the injured region, intensity, duration and frequency of care management, delay post-stroke before the beginning of treatment, combination of different approaches, as well as prognostic and efficacy criteria. An encouraging perspective for the future is the combination of several types of approaches, which would be largely facilitated by the improvement of fundamental knowledge on neglect mechanisms, which could in the future refine the choice for the most appropriate treatment(s) for a given patient.
1
Introduction
Hemi-spatial neglect syndrome has a particular place among pathologies affecting the integration of spatial information. It constitutes a spatial cognition disorder frequently observed after stroke. This disorder characterized by a deficit in the use of and awareness of one side of space is a long-lasting phenomenon, most often occurring after a right hemispheric injury, noticeably in the parietal region and specifically around the inferior parietal lobule, i.e. around a region playing the role of a multi-sensory and sensorimotor interface between spatial perception and action.
This multi-faceted syndrome associates a deficit in taking into account sensory information stemming from the part located on the contralateral side of the brain injury, a change in orientation with reactions and actions directed towards that side, as well as behavioral symptoms resulting from the altered awareness of the patient regarding these disruptions.
Taking into account this diversity and all the clinical characteristics is essential for a more appropriate evaluation and adapted care management rehabilitation. This is even more relevant since spatial cognition disorders, and above all hemi-spatial neglect, are at the source of major limitations of activities and constitute a predictive factor of a poor functional prognosis, delaying the recovery of cognitive and motor autonomy . Taking into account this syndrome is thus a real therapeutic challenge in the rehabilitation care management of these patients, to try and reduce the disability and improve the prognosis.
Two main theoretical tendencies can be differentiated in unilateral neglect rehabilitation: top-down and bottom-up approaches (see reviews in ). More recently, transversal approaches have been developed, targeting more specifically impairments with no spatial laterality frequently associated to spatial neglect.
Among the therapeutic axes recently developed, some of them appear particularly promising (see review in ). The use of non-invasive brain stimulation in patients has been widely reported for its high therapeutic relevance (see ), especially in the framework of research on spatial neglect: Transcranial Magnetic Stimulation (TMS) and transcranial Direct Current Stimulation (tDCS) are used to improve symptoms of patients with visuo-spatial disorders.
Non-invasive brain stimulation using Transcranial Magnetic Stimulation (TMS) and transcranial Direct Current Stimulation (tDCS) can be used not only as a diagnostic research tool to explore pathophysiological aspects of spatial neglect, but also to improve symptoms.
The objective of this literature review is to provide an overview of the various paradigms used in this context, for exploratory and/or therapeutic uses, in the framework of single-case studies or randomized, controlled trials.
This literature review focused on publications in the English language indexed in PubMed pertaining to the use of one of these techniques for evaluation purposes or to improve symptoms in patients presenting post-stroke hemi-spatial neglect. For the search, the following keywords were combined: “TMS or tDCS or transcranial magnetic stimulation or transcranial direct current stimulation” and “spatial neglect or visual neglect or hemi-spatial neglect or visuo-spatial neglect”. By reading the titles and abstracts, original articles or reviews of the existing literature were selected. References of selected articles were also studied in order to find additional ones. Table 1 lists the different studies selected pertaining to the clinical use of one or the other techniques, according to the type of symptoms improved (deficits) and/or the functional impact in terms of activity limitations.
| Deficits | |
| Visuomotor tasks (line bisection, line crossing, drawing tests) | Oliveri et al., 2001 |
| Brighina et al., 2003 a | |
| Shindo et al., 2006 a | |
| Song et al., 2009 a | |
| Lim et al., 2010 a | |
| Koch et al., 2012 a | |
| Cazzoli et al., 2012 a | |
| Kim et al., 2013 a | |
| Kim et al., 2014 a | |
| Ko et al., 2008 | |
| Sparing et al., 2009 | |
| Sunwoo et al., 2013 | |
| Brem et al., 2014 a | |
| Visual and verbal tasks (object description, image description) | Koch et al., 2008 |
| Cazzoli et al., 2012 a | |
| Tactile extinction | Oliveri et al., 1999 |
| Visual extinction | Nyffeler et al., 2009 |
| Visual perception | Cazzoli et al., 2012 a |
| Kim et al., 2013 a | |
| Sparing et al., 2009 | |
| Activity limitations | |
| Barthel index | Shindo et al., 2006 a |
| Kim et al., 2013 a | |
| Behavioral BIT | Koch et al., 2012 a |
| Reading | Cazzoli et al., 2012 a |
| Activities of daily living | Shindo et al., 2006 a |
| Cazzoli et al., 2012 a | |
| Kim et al., 2013 a | |
| Brem et al., 2014 a | |
| Participation limitations (disability) | |
| No studies |
2
Facilitating or inhibiting effects of non-invasive brain stimulation
According to the protocol used, brain stimulation can have opposite effects on the underlying brain tissues: low-frequency rTMS (1 Hz), continuous Theta Burst Stimulation (TBS) and cathodal tDCS decrease cortical excitability, whereas high-frequency rTMS (5 Hz) intermittent Theta Burst Stimulation and anodal tDCS seem to increase cortical excitability with facilitating effects .
2
Facilitating or inhibiting effects of non-invasive brain stimulation
According to the protocol used, brain stimulation can have opposite effects on the underlying brain tissues: low-frequency rTMS (1 Hz), continuous Theta Burst Stimulation (TBS) and cathodal tDCS decrease cortical excitability, whereas high-frequency rTMS (5 Hz) intermittent Theta Burst Stimulation and anodal tDCS seem to increase cortical excitability with facilitating effects .
3
Rationale of use in the context of spatial neglect
To understand the different types of modulation of visuo-spatial functions via non-invasive brain stimulation, it seems relevant to briefly review the attention networks involved in visuo-spatial neglect and clarify the concept of inter-hemispheric competition. Visuo-spatial neglect is more and more defined as resulting from the interruption of the fronto-parietal attention networks, especially those located in the right hemisphere . Furthermore, as suggested by Kinsbourne , both parietal cortices, right and left, exert between themselves a reciprocal inter-hemispheric inhibition. Thus, after a parietal injury to the right hemisphere, we observe not only a decreased activity in this injured region, but also a disinhibition of the contralateral left hemispheric region. This inter-hemispheric of the left hemisphere worsens the tendency of patients with spatial neglect to only pay attention to the right side and disregard the left side. This has been underlined by clinical observations and functional imaging data. Vuilleumier et al. reported a unique case of one patient with two successive sequential lesions the first on the right hemisphere followed by a second on the left hemisphere, the first lesion led to severe left spatial neglect, which resolved itself after the onset of the second lesion. The longitudinal follow-up via fMRI of patients with spatial neglect highlighted an initial over-activation on the healthy side. The clinical recovery of spatial neglect was associated with an increased activation of certain right hemispheric regions, but also activation changes on the healthy left side, leading to a reduction of the inter-hemispheric imbalance. The recovery of spatial neglect-related attention deficits thus seems correlated to a reactivation and a recalibration of functional and structural activity within the fronto-parietal networks involved.
Today’s approaches on neglect modulation are thus essentially based on this neurophysiological concept of inter-hemispheric inhibition, suggesting an over-activation of the contralesional hemisphere due to the decreased inhibiting influences of the injured hemisphere.
Based on this notion, 3 approaches seem valid ( Fig. 1 ): stimulation of the injured right hemisphere, inhibition of the hyperactive left hemisphere or a combination of both. To date, most studies on non-invasive brain stimulation targeting neglect have chosen to inhibit the left hemisphere, but the facilitating protocols to increase the functions of ipsilesional neural circuits merit further development. One of the potential barriers to this latter approach, especially with TMS, could be the increased risk of seizure.
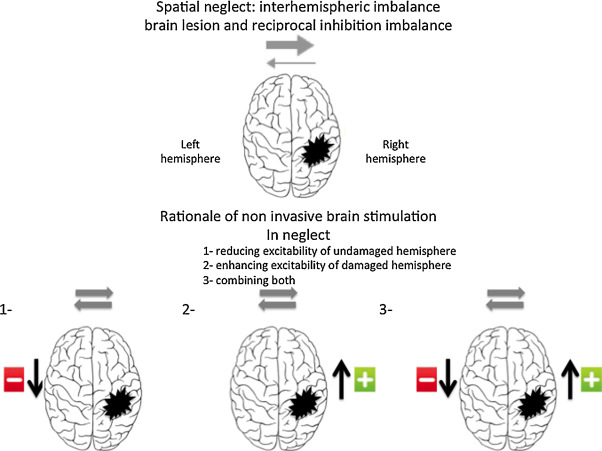
The main studies are listed in Table 1 .
Table 2 (TMS) and Table 3 (tDCS) review the different studies retained for this review, with a brief description of the type of study, stimulation parameters used, evaluation tests as well as main results reported.
| Authors, year | Type of study | Stimulation parameters | Positioning method | Patients | Delay | Evaluations | Significant results |
|---|---|---|---|---|---|---|---|
| Oliveri et al., 1999 | Controlled study (stimulation site) | Single pulse TMS F3 F4 and P3 P4, unaffected hemisphere | 10/20 EEG system | 14 RBD 14 LBD | 1–4 m | Contralateral extinction | Extinction reduction only for RBD patients and F stimulation |
| Oliveri et al., 2001 | Controlled study (rTMS vs sham) | rTMS P5 P6 unaffected hemisphere | 10/20 EEG system | 5 RBD 2 LBD | 1–48 w | Task length judgment | Reduced ipsilesional attentional bias RBD and LBD patients |
| Brighina et al., 2003 a | Uncontrolled pilot study | 1 Hz rTMS P5 unaffected hemisphere 7 sessions, 2 w | 10/20 EEG system | 3 RBD N+ | 3–5 m | Landmark Clock drawing Line bisection | Improvement in the 3 tasks, maintained 15 days after |
| Shindo et al., 2006 a | Uncontrolled pilot study | 0.9 Hz rTMS P5 unaffected hemisphere 6 sessions, 2 w | 10/20 EEG system | 2 RBD N+ | 6 m | BIT MMSE or HDS-R BRS Barthel Index | BIT improvement, maintained at 6 weeks |
| Koch et al., 2008 | Experiment 1 Controlled study | ppTMS conditioning left PPC (P3) – test M1 | 10/20 EEG system | 12 RBD N+ 8 RBD N− 10 healthy controls | 91.6d 83.9d | left PPC-M1 effects: MEPs amplitude | Pathologically increased left PPC-M1 effects observed selectively in the N+ group, correlated with severity of neglect |
| Koch et al., 2008 | Experiment 2 Controlled study | 1 Hz rTMS left PPC (P3) | 10/20 EEG system | 10 RBD N+ 5 RBD N− | MEPs amplitude Visual chimeric objects naming | Normalization of the abnormal left PPC-M1 influences in N+ Improvement of the experimental visual chimeric test | |
| Song et al., 2009 a | Randomized study, controlled vs sham | 0.5 Hz rTMS left PPC (P3) 2 groups: | 10/20 EEG system | 14 RBD N+ | 21–60d | Line bisection Line cancellation | Improvement in both tasks, stable by 2 weeks |
| rTMS | 7 | ||||||
| sham | 7 | ||||||
| 20 sessions (2/d, 2 w) | |||||||
| Nyffeler et al., 2009 | Randomized controlled study | cTBS left PPC (P3) | 10/20 EEG system | 11 RBD N+ | 0.4–36.1 m | Visual perception task (PVT) | Improvement of targets’ perception on the left side Decreased RT for left-sided targets |
| 4 conditions: | 5 | ||||||
| no intervention | patients/exp | ||||||
| 2 sham trains | |||||||
| 2 TBS trains | |||||||
| 4 TBS trains | |||||||
| Lim et al., 2010 a | Comparative open pilot study | 1 Hz rTMS left PPC (P5) | 10/20 EEG system | 14 RBD N+ | Schenkenberg test Albert test | Improvement in line bisection | |
| 2 groups: | |||||||
| rTMS + BT | 7 | 61.9d | |||||
| rTMS immediately prior to BT | |||||||
| BT 30 mn top-down approach | 7 | 139.0d | |||||
| Koch et al., 2012 a | Randomized study, controlled vs sham, double-blind | cTBS left PPC (P3) 10 sessions (1/d, 2 w) | Neuronavigation system | 18 RBD N+ | 25–100d | left PPC-M1 connectivity (MEPs amplitude) BIT (C + B) | Reduction of hyper-excitability of LH parieto-frontal circuits Improvement of neglect symptoms (BIT) |
| Cazzoli et al., 2012 a | Randomized study, controlled vs sham, double-blind | cTBS left PPC (P3) | 10/20 EEG system | 24 RBD N+ | 26.63d | Visual perception task (PVT) | Improvement of detection of left-sided visual targets |
| 3 groups: | |||||||
| cTBS-sham | 8 | Shape cancellation test | Improvement in the paper-pencil assessment | ||||
| sham-cTBS | 8 | Picture scanning test | Improvement in the activities of daily living | ||||
| no stim | 8 | Texts reading CBS | |||||
| Kim et al., 2013 a | Randomized study, controlled vs sham, double-blind | rTMS | 10/20 EEG system | 27 RBD N+ | 14.2–16.4d | MVPT | Improvement in line bisection (high frequency vs sham) Improvement of K-MBI score in the 2 rTMS groups |
| 3 groups | |||||||
| 1 Hz left PPC (P3) | 9 | Line bisection | |||||
| 10 Hz right PPC (P4) | 9 | Star cancellation | |||||
| sham | 9 | CBS | |||||
| 10 sessions (1/d, 2 w) | K-MBI | ||||||
| Kim et al., 2015 a | Randomized controlled study | rTMS 1 Hz | 10/20 EEG system | 34 RBD N+ | 3–45 m | Line bisection Letter cancellation Ota’s task | Improvement in all tasks with 10 sessions vs single session |
| Left PPC (P3) | |||||||
| 2 groups: | |||||||
| 1 session | 19 | ||||||
| 10 sessions | 15 | ||||||
| (1/d, 2 w) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








