Despite major advances in total shoulder arthroplasty, management of severe posterior glenoid bone loss remains controversial. Several companies have provided alternative treatment options for type C glenoids associated with posterior subluxation of the humeral head. However, preoperative planning, proper selection of glenoid size, and recognition of the operative pitfalls are crucial for successful outcomes. A review of the literature and presentation of the surgical technique for the management of severe posterior glenoid bone loss are presented.
Key points
- •
Despite major advances in total shoulder arthroplasty, management of severe posterior glenoid bone loss remains controversial.
- •
Several companies have provided alternative treatment options for type C glenoids associated with posterior subluxation of the humeral head.
- •
Preoperative planning, proper selection of glenoid size, and recognition of the operative pitfalls are crucial for successful outcomes.
Introduction
With improvements in component design, technology, and surgical technique, total shoulder arthroplasty (TSA) is a highly successful surgical procedure for glenohumeral arthritis. However, lucent lines around the glenoid component and glenoid component loosening remain a major concern. Preoperative recognition of glenoid morphology and proper surgical planning are key factors for successful outcomes after surgical treatment of glenohumeral arthritis.
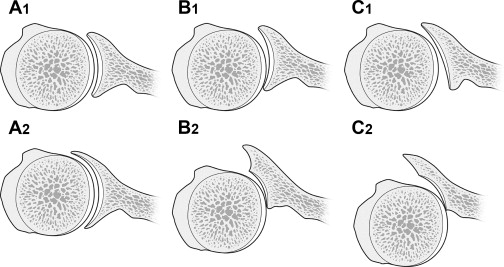
The glenoid classification by Walch and colleagues has been widely accepted for preoperative planning. Walch and colleagues classified glenoid morphology in primary glenohumeral arthritis into 5 types: In type A1, the humeral head is centered and minor glenoid erosion occurs centrally. In type A2, the head is centered and major glenoid erosion occurs centrally. In type B1, the humeral head is subluxated posteriorly without glenoid erosion. In type B2, the humeral head is subluxated posteriorly and the glenoid has posterior erosion with the development of biconcavity. In type C, there is glenoid dysplasia or hypoplasia (retroversion >25°) with or without posterior wear. Among these types, the operative treatment of type B2 and C remains most controversial.
The purpose of this article is to address the present operative strategies for B2 and C glenoids and to highlight the surgical technique and its pitfalls.
Surgical treatment of b2 glenoid
Several treatment strategies have been reported for type B2 glenoids, such as asymmetric reaming, bone grafting, augmented components, and reversed TSA.
Asymmetric Reaming and Glenoid Resurfacing
With asymmetric reaming, it can be difficult to re-create normal glenoid version in cases of severe glenoid retroversion without removing substantial anterior bone. Sabesan and colleagues demonstrated that correction of moderate to severe glenoid retroversion by asymmetric reaming cannot always be done with the use of a standard component, and if it is done, it will result in greater medialization of the joint line. In addition, Clavert and colleagues have demonstrated that correction of greater than 15° of retroversion is not possible without violating the anterior subchondral bone or the glenoid vault with the anchoring points. Gillespie and colleagues also have demonstrated that a 15° deformity has only a 50% chance of successful correction by anterior, eccentric reaming in a cadaveric model.
Basic study and clinical results of asymmetric reaming and glenoid implantation have been mixed. Most recently, Walch and colleagues have demonstrated that violation of subchondral bone can lead to early glenoid radiolucency and failure. Study of the use of a standard glenoid component in the setting of a biconcave glenoid demonstrated high rates of complications. Gillespie and colleagues demonstrated that correction of as little as 10° of posterior glenoid wear by preferential anterior glenoid reaming results in significant narrowing of the glenoid anteroposterior distance by their cadaveric study. They also demonstrated that corrective glenoid reaming for wear of greater than 10° results in peg penetration in most glenoids and downsizing of glenoid size for most glenoids. On the other hand, Gerber and colleagues showed that asymmetric reaming resulted in correction of posterior humeral subluxation in 21 of 23 patients (91%). Similarly, Habermeyer and colleagues showed that, with asymmetric reaming and soft tissue balancing, the humeral head was maintained in a recentered position following surgical correction of glenoid morphology.
Ream and Run
“Ream and Run” is a specific procedure in which a humeral arthroplasty is performed for active patients in conjunction with concentric reaming of the glenoid bone to spherical concavity with a diameter of curvature 2 mm greater than that of the prosthetic humeral head. Clinton and colleagues demonstrated that the ream and run can offer similar functional recovery to patients with TSA, although the time to recovery may be longer. Matsen and colleagues presented that the ream and run substantially corrected the glenoid type in conjunction with B2 glenoid on the axial view radiographs. Gilmer and colleagues concluded that the procedure appears to be best suited for older male patients with reasonable preoperative shoulder function without prior shoulder surgery, as an analysis of 176 consecutive cases after the procedure based on patient self-assessment, like the simple shoulder test. They also concluded that the type of glenoid had no significant effect on the outcome, and their patients had no problems with posterior glenohumeral instability, although a substantial number of the glenoids were posteriorly eroded and the humeral head was displaced into the posterior aspect of a biconcavity.
Bone Grafting
Studies of clinical and radiographic results of primary total shoulder replacement with an all-polyethylene glenoid component and autologous humeral head graft augmentation have demonstrated mixed results. Neer and Morrison reported excellent results in 16 patients and satisfactory results in 3 patients, and no revision surgeries. No glenoid loosening or migration had occurred at a minimum follow-up of 2 years (average, 4.4 years). Steinmann and Cofield reported that at a mean of 5 years postoperatively, 23 of 28 (82%) patients had satisfactory results after concomitant bone grafting and TSA. However, 15 patients (54%) demonstrated some degree of radiographic lucency, and 3 glenoids were radiographically loose at an average follow-up of 5.3 years. Hill and Norris reviewed 17 TSAs at a mean of 70 months postoperatively that had undergone concomitant bone grafting to address glenoid erosion. Five (29%) of the grafts failed, resulting in requiring revision as a result of instability (2 patients). Sabesan and colleagues reported that 10 of the 12 patients had graft incorporation without any resorption and 2 patients had minor bone graft resorption. Broken screws occurred in 2 of these 10 cases. Two patients, both of whom required revision surgery, had failure of fixation and of graft incorporation. These studies indicate that posterior subluxation can be corrected with glenoid bone grafting, but that the technique may be difficult to perform, generates inconsistent results, and may result in hardware complications and late graft failure in some cases.
Augmented Polyethylene Glenoid
When posterior bone loss is between 3 and 9 mm on the axial view, an augmented component can be used. It has been shown in a 3-dimensional surgical simulation that the use of an augmented component can allow complete correction of retroversion and minimize the effect of medialization. Similarly, compared with a stepped, augmented component, retroversion greater or equal to 20° has been shown to make it difficult to place a pegged glenoid component perpendicular to the plane of the scapula by asymmetric reaming without center peg perforation. In the senior author’s practice (G.R.W.), a posteriorly augmented glenoid component is typically preferred in the setting of a B2 glenoid with between 3 mm and 9 mm of posterior bone loss.
Reverse Arthroplasty
At early follow-up, Mizuno and colleagues reported successful clinical and radiographic results of reverse TSA for the treatment of primary osteoarthritis in patients with a biconcave glenoid without rotator cuff insufficiency. Despite this, reverse arthroplasty carries its own particular risks of complications and shortcomings. More data will be required to determine the indications of reverse arthroplasty in patients with posterior subluxation and posterior glenoid bone loss and an intact rotator cuff.
Surgical treatment of type C glenoids
Walch and colleagues defined type C glenoid morphology as glenoid retroversion of greater than 25°. This type is most commonly associated with congenital or dysplastic development. Glenohumeral arthritis secondary to glenoid dysplasia appears to be relatively uncommon. Although type C glenoid is thought to be rare, some reports indicate that it may be more common than previously speculated and shoulder arthroplasty has proven to be a viable option. However, the choice of treatment options still remains controversial. Bonnevialle and colleagues reported marked improvement in pain scores, function, and outcome measures at a minimum 2-year follow-up in 9 patients treated with hemiarthroplasty for dysplasia. The authors concluded that hemiarthroplasty is a reliable management option for this patient population. On the other hand, Sperling and colleagues reported that 3 of 4 patients treated with hemiarthroplasty underwent revision to TSA as a result of glenoid arthrosis at 16 months, 20 months, and 34 months. They concluded that hemiarthroplasty alone appears to be an unsatisfactory option for the treatment of dysplastic glenoid. Edwards and colleagues demonstrated significant improvement in outcome measures at a mean of 37 months in 15 patients with primary osteoarthritis and type C glenoid treated with hemiarthroplasty or total arthroplasty. These successful results might be due to their criteria that glenoid resurfacing was 15 mm or greater of glenoid bone depth on axial computed tomography (CT). However, in cases of severe dysplasia, the amount of bone available for fixation may be inadequate for standard fixation options. In these patients, the use of an inset bone-sparing glenoid component with a single, short peg may be helpful to avoid cortical penetration.
Among shoulders with type C glenoids, there are certain shoulders whose humeral head does not remain centered on the surface of the glenoid and is posteriorly subluxated with regard to the surface of the glenoid, resulting in biconcavity; this may be defined as C2 glenoids (Michael Wiater, MD, personal communication, 2011) ( Fig. 1 ). The posterior rotator cuff in a C2 glenoid is likely relatively short by virtue of the shorter distance between the origin and insertion of the muscles since birth. Hence, patients with C glenoids may lose internal rotation motion if correction of retroversion to neutral version is performed. Therefore, the author aims for correction of preoperative subluxation to the point where the humeral head is recentered on the surface of the native glenoid without necessarily completely correcting the version to neutral. Therefore, in the senior author’s (G.R.W.) practice, a C2 glenoid (ie, biconcave) with 9 mm or greater of posterior bone loss is also an indication for a posteriorly augmented, stepped component, with the idea of correcting the biconcavity without complete correction of retroversion. Type C glenoids without posterior subluxation (C1) are less often corrected with an augmented component.
Surgical Indications
In the case of shoulders with 3 mm or greater of posterior wear, asymmetric reaming of the anterior glenoid (high side) is performed and a standard, pegged glenoid component is used. In shoulders with 5 mm of posterior wear, anterior reaming of 2 mm is undertaken and a 3-mm stepped component is used. In shoulders with 7 mm of posterior wear, anterior reaming of 2 mm is undertaken and a 5-mm stepped component is used. In shoulders with 9 mm of posterior wear, anterior reaming of 2 mm is undertaken and a 5-mm stepped component is used. In the cases of shoulders with greater than 9 mm of posterior wear, bone grafting is often indicated and a stepped component may not be appropriate. In rare cases of shoulders with inadequate peripheral or central bone to allow for placement of a pegged glenoid component (type C1 glenoids or revisions) or those thought to be at high risk for bone graft resorption and failure, a Mini Glenoid (Arthrosurface, Franklin, MA, USA) may be used.
These recommendations are only guidelines based on experience. In addition, it is important to note that the glenoid deficiency is 3-dimensional and includes differences in inferior tilt as well as anterior and posterior role. The 3-dimensional aspect of the deformity is difficult to appreciate without 3-dimensional CT imaging and application of software that can assist the surgeon in the appropriate placement of the component in all 3 planes. Multiple software programs currently exist to help achieve anatomic correction, but an in-depth discussion of this topic is beyond the scope of this article.
Surgical Technique
A standard deltopectoral approach is used. The superficial and deep dissections have been described in detail previously. Current preference for subscapularis management is a lesser tuberosity osteotomy.
Preoperative Planning, Sizer Pin Guide Placement, and Sizing
Planned step height is determined based on the preoperative CT scan. If a preoperative preparation software system is available to the surgeon, it is helpful—particularly in cases of severe deformity. Iannotti and colleagues demonstrated that use of a preoperative planning tool based on CT scans provides more accurate placement of the component. Experience with these types of planning tools is currently not extensive but is increasing. In the future, this type of preoperative and intraoperative assistance in the placement of glenoid components in cases of glenoid bone loss is likely to be common.
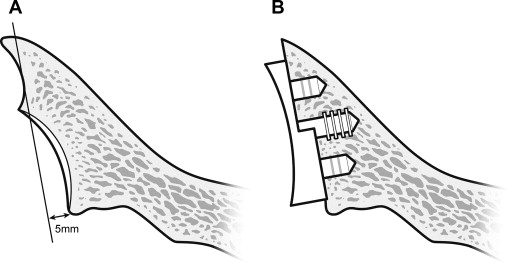
In the absence of a preoperative planning tool, it is important for the surgeon to use the 3-dimensional CT scan to appreciate the glenoid deformity in all 3 planes. The most obvious plane is the axial plane. However, the coronal and sagittal planes are also important. Typically, posteriorly biconcave glenoids also demonstrate variable superior tilt in the coronal plane and posterior tilt in the sagittal plane. If, for example, the preoperative CT shows 5 mm of posterior bone loss on the axial cuts, a sizer pin guide with a 5-mm posterior buildup is selected so that, with 2 mm of anterior reaming, a 3-mm posterior step can be created for insertion of a 3-mm posteriorly augmented component ( Fig. 2 ). The surgeon should also be cognizant of any superior or posterior tilt seen on the preoperative CT scan and adjust the direction of the pin accordingly.