Abstract
Introduction
Post-stroke aphasia makes it difficult to assess cognitive deficiencies. We thus developed the CASP, which can be administered without using language. Our objective was to compare the feasibility of the CASP, the Mini Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA) in aphasic stroke patients.
Material and methods
All aphasic patients consecutively admitted to seven French rehabilitation units during a 4-month period after a recent first left hemispheric stroke were assessed with CASP, MMSE and MoCA. We determined the proportion of patients in whom it was impossible to administer at least one item from these 3 scales, and compared their administration times.
Results
Forty-four patients were included (age 64 ± 15, 26 males). The CASP was impossible to administer in eight of them (18%), compared with 16 for the MMSE (36%, P = 0.05) and 13 for the MoCA (30%, P = 0.21, NS). It was possible to administer the CASP in all of the patients with expressive aphasia, whereas the MMSE and the MoCA could not be administered. Administration times were longer for the CASP (13 ± 4 min) than for the MMSE (8 ± 3 min, P < 10 −6 ) and the MoCA (11 ± 5 min, P = 0.23, NS).
Conclusion
The CASP is more feasible than the MMSE and the MoCA in aphasic stroke patients.
Résumé
Introduction
L’aphasie post-AVC rend difficile l’évaluation des autres fonctions cognitives. Nous avons élaboré le CASP qui peut être passé sans utiliser le langage. Nous avons comparé les faisabilités du CASP, du Mini Mental State Examination (MMSE) et de la Montreal Cognitive Assessment (MoCA) chez des patients cérébro-lésés gauches vasculaires aphasiques.
Patients et méthodes
Tous les patients hospitalisés sur une période de quatre mois dans sept services de rééducation français pour premier AVC hémisphérique gauche récent avec aphasie ont été évalués grâce aux trois batteries. Nous avons estimé le pourcentage de patients chez qui la passation d’au moins un item de ces échelles était impossible et comparé leur temps de passation.
Résultats
Sur quarante-quatre patients inclus (64 ± 15 ans, 26 hommes), 8 (18 %) CASP étaient non réalisables, contre 16 (36 %) MMSE ( p = 0,05) et 13 (30 %) MoCA ( p = 0,21, NS). Tous les patients avec troubles isolés de l’expression ont pu bénéficier du CASP, aucun des autres batteries. Les temps de passation étaient était plus longs pour le CASP (13 ± 4 min) que pour le MMSE (8 ± 3 min, p < 10 −6 ) et la MoCA (11 ± 5 min, p = 0,23, NS).
Conclusion
En termes de faisabilité, le CASP est mieux adapté aux patients aphasiques que le MMSE et la MoCA.
1
English version
1.1
Introduction
All cognitive functions can be affected post-stroke (language, attention, memory, praxis, gnosis, executive functions) and their evaluation is important on many levels. Their screening leads to early management by a specialized therapist (speech and language therapist, neuropsychologist, occupational therapist); repeating this evaluation over time enables to refine the prognosis on the medium term, especially in the perspective of returning home or back to work.
The presence of language disorders makes it more difficult to evaluate and manage the other cognitive disorders. Therefore, it is not easy to analyze the spatiotemporal orientation or screen for memory disorders without using language.
To this day, screening tests for quantifying cognitive disorders, generic or dedicated to stroke patients, are not suited to aphasic patients, because they include items that require language-based answers. The best known battery of tests is the Mini Mental State Examination (MMSE) , recommended by the Higher Health Authority in France to screen for dementia and very much used by young medical residents. Even though certain MMSE items require some verbal answers and are thus incompatible to patients with severe language impairments, it remains the most commonly used scale in France for stroke patients. Among the scales best suited to these patients, one can find the R-CAMCOG (Rotterdam-CAMCOG, modified version of the Cambridge Cognitive Examination) and the MoCA (Montreal Cognitive Assessment) . Better results were in fact reported for the MoCA vs. the MMSE, especially for executive functions, memory and attention disorders in terms of sensitivity and specificity . However, the MMSE and MoCA seemed similar in the acute phase post-stroke for predicting the cognitive status on the medium term . If the choice of items for the R-CAMCOG and MoCA appears quite relevant for the evaluation of cognitive impairments post-stroke, their design remains problematic since some items, i.e. abstraction, orientation or memory, require a verbal answer. Another battery, the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), showed a good reliability to predict cognitive prognosis post-stroke on the medium term when administered upon admission in a PM&R unit . But here again, patients with language impairments are unable to answer all items. As an example, the items of two of the most used scales, MMSE and MoCA are outlined in Table 1 along with their potential feasibility in case of severe language impairments.
| In case of severe verbal expression impairments | MMSE | MoCA |
|---|---|---|
| Achievable items | Language Copy of drawings | Visuo-spatial-executive Naming Language |
| Non-achievable items | Orientation Memory Attention and calculation Recall | Memory Attention Abstraction Recall Orientation |
In fact this leads to two main consequences:
- •
in daily clinical practice, screening and precisely quantifying cognitive impairments (outside of language) in aphasic patients cannot be based on these batteries of tests when administered by a “non-expert”. It is only possible when administered by a speech or language therapist, neuropsychologist or experimented physician, and not a medical resident who is at the forefront of the care management pathway;
- •
aphasic patients are regularly excluded from research protocols on the recovery of cognitive and/or physical functions post-stroke, thus limiting the generalization of such studies .
However, it is possible to evaluate at least roughly all cognitive functions without using language as support. This implies reformulating the instructions in different manners, simplifying them to the maximum or using strictly visual tests. For example, a visual memory test with a free recall-recognition consists in having a first stage where the patient memorizes the images and then having him or her recognize these images among other ones, which are so-called “distractor” images. We can also test praxis by imitation rather than on command (even though some dissociation may exist). This type of evaluation requires a certain know-how and sometimes a longer administration time, not compatible with a quick screening at the “patient’s bedside”.
In 2011, a group of French experts got together to elaborate an assessment scale for evaluating cognitive disorders in post-stroke patients compatible with the presence of severe language impairments: the Cognitive Assessment scale for Stroke Patients (CASP). This group included 8 PM&R physicians or neurologists, 7 speech and language therapists and 6 neuropsychologists from 5 different University-Hospital PM&R teams specialized in neurology, one hospital neurology rehabilitation department and one PM&R center.
1.1.1
Requirements
The battery of tests had to:
- •
enable the screening and quantifying of cognitive disorders post-stroke (language, praxis, short-term memory, temporal orientation, spatial/visuo-construction neglect, executive functions);
- •
be usable even in the presence of severe verbal expression impairments;
- •
be suited to daily clinical practice, at the “patient’s bedside”.
Adapting cognitive scales to aphasic patients (especially with left hemisphere damage) meant the preferential use of visual aids. However, using visual aids might impair the administration of the tests in case of spatial neglect (especially in patients with right hemisphere lesions). Thus, we took into account the possible presence of language impairments and left spatial neglect to elaborate the CASP. In fact, words or images to be presented to patients were systematically placed on the right side of the test sheet and/or ordered in columns rather than in lines.
It is important to underline that the CASP was not meant to be used in patients with severe comprehension disorders. Since then, our experience with this tool has shown that if we only consider the “verbal comprehension” facet, a minimum score of 3 on the aphasia severity test of the Boston Diagnostic Aphasia Examination (BDAE) is a prerequisite element.
1.1.2
Development of the CASP
A literature review (on Pubmed, with no date range) allowed the identification of neuropsychological scales and batteries of tests, which included some items that were relevant to the CASP. Thus, the work database put together included several tools, all validated or commonly used in evaluating cognitive impairments: scales validated in post-stroke, generic evaluation scales or specific to cognitive impairments, “classic” clinical guidelines established upon a consensus of experts . In the end, several of these tools were identified or listed in reference manuals on neuropsychological batteries of tests in French and English .
Two successive administrations of the scale on two groups of thirty patients (left and right hemisphere stroke patients recruited within the participating PM&R units, see below) led to the evolution of the CASP towards a second, and then a third version which was retained as the final version . The changes mainly concerned the design of the items and clarity of the instructions.
The CASP includes nine items evaluating a total of 6 cognitive functions: language, praxis, short-term memory, temporal orientation, spatial/visuo-construction neglect and executive functions ( Table 2 and Appendix ). Each function is quoted on 6 points; the maximum total score is 36 points. The results from the CASP administration can be presented either as a profile (e.g. “6/5/4/5/3/4”) for a qualitative analysis of the impairments, or as a global score.
| Language (6 points) | |
| Item 1 (3 points) Verbal expression | Naming images from the Snodgrass picture set |
| Item 2 (3 points) Verbal comprehension | Simple and complex orders using common objects or body parts |
| Visuo-construction and unilateral spatial neglect (6 points) | |
| Item 3 (4 points) Reproducing a cube | Used alone in daily practice and integrated within the BEC-96 (Cognitive Evaluation Scale) |
| Item 6 (2 points) Bisection of a 20-cm line | Test validated individually and in the framework of the BEN scale (Neglect Evaluation Battery) |
| Executive functions (6 points) | |
| Item 4 (2 points) Graphic series | Used in clinical practice and recommended by the French College of Neurology Professors |
| Item 5 (4 points) Conflict resolution test and go-no go | Adapted from the FAB (Frontal Assessment Battery) |
| Memory (6 points) | |
| Item 7 (6 points) Image recall | Recall-recognition of images used in item 1, among distractor ones. Images from the Snodgrass picture set |
| Praxis (6 points) | |
| Item 8 (6 points) Praxis tests | Common clinical evaluations and tests from the “short battery for evaluation of gestural praxis from the expert group on praxis CMRR Île-de-France South” |
| Orientation (6 points) | |
| Item 9 (6 points) Calendar | Reading a calendar with multiple choices for the year, month, day, day of the month and day of the week |
The validation of the CASP (validity, reliability and sensitivity to change) on a population of stroke patients (with or without aphasia) is ongoing in the framework of the “French Clinical Research Program 2012” (PHRC). The objective of the present work was to evaluate the relevance of the CASP, in terms of feasibility, compared to the MMSE and MoCA in aphasic patients after left-sided stroke.
1.2
Patients and methods
In each participating PM&R department and over a period ranging from two to four months (according to the department’s recruitment), all patients hospitalized for a recent primary left-sided stroke with aphasia were considered for inclusion. They benefited from the systematic administration of the CASP, MMSE and MoCA. Then we:
- •
estimated the percentage of patients for whom one or several items of these three scales was not achievable;
- •
compared the mean administration time.
1.2.1
Inclusion criteria
The criteria were:
- •
patients hospitalized in a PM&R unit within one hundred days after a primary stroke affecting the left hemisphere and presenting with language impairments;
- •
no restrictions in terms of age, severity of the cognitive disorders and severity of aphasia (BDAE aphasia severity item between 0 and 5).
1.2.2
Non-inclusion criteria
The criteria were:
- •
disorders of consciousness;
- •
patients not speaking French;
- •
history before the stroke of cognitive, psychotic or visual disorders not compatible with reading.
1.2.3
Administrations of the three batteries
According to the local settings, the batteries could be administered by a physician, medical resident, speech and language therapist or neuropsychologist. The order in which they were administered was predetermined by a random draw. For each patient, one sole examiner had to administer all three batteries.
1.2.4
Collected data
Besides scores and administration time of the three batteries, we collected data on general demographics, stroke characteristics and BDAE aphasia severity score.
1.2.5
Data analysis
After having verified that all conditions were met we used the variance analysis to compare means, the Chi 2 test to compare percentages and the Pearson ( r ) correlation coefficient for the correlations. Their non-parametric equivalent was used when the validity conditions were not met. Concordance between scores was based on the Intraclass Correlation Coefficient (ICC). All data were analyzed with the Number Cruncher Statistical System software .
1.3
Results
Forty-four patients were included, mean age 64 ± 15 years ( r : 22–89), including 26 men (59%), 43 right-handed (98%) and 1 ambidextrous (2%). Damage strictly concerned the left hemisphere in 43 patients (98%) and was bilateral in 1 patient (2%), the stroke was ischemic for 35 patients (80%), hemorrhagic for 7 (16%), and ischemic and hemorrhagic in 2 cases (5%). Time interval since stroke was 42 ± 22 days ( r : 11–100). The repartition of patients according to the BDAE scores of 0, 1, 2, 3, 4 and 5 was respectively: 8, 9, 12, 8, 4 and 3, (median = 2).
Results of the administration of the three batteries are listed in Table 3 . The number of impossible administrations were significantly less important for the CASP (8, 18%) than for the MMSE (16, 36%, P = 0.05). It was less important (not statistically significant) than for the MoCA (13, 30%, P = 0.21). No administration failure was observed for the CASP in the three patients with isolated verbal expression impairments, whereas they were unable to complete the other two batteries.
| Test | Incomplete test administrations | Complete test administration | |||
|---|---|---|---|---|---|
| Number | Aphasia | Number | Scores | Times | |
| CASP (/36) | 8 (18%) | 8 VC, 0 V | 36 (82%) | 24 ± 9 (4–36) | 13 ± 4 (7–20) |
| MMSE (/30) | 16 (36%) | 13 VC, 3 V | 28 (64%) | 14 ± 8 (2–27) | 8 ± 3 (5–15) |
| MoCA (/30) | 13 (30%) | 10 VC, 3 V | 31 (70%) | 10 ± 8 (0–27) | 11 ± 5 (5–22) |
Mean scores were 24 ± 9/36 for the CASP, 14 ± 8/30 for the MMSE and 10 ± 8/30 for the MoCA, with a strong correlation between the three ( r > 0.75, P < 10 −5 for the three comparisons, two by two). However, only the concordance between the MMSE and MoCA was good (ICC = 0.83, [0.70–0.91]). It was medium between the CASP (total score brought down to 30) and MMSE (ICC = 0.60, [0.36–0.76]), and poor between the CASP and MoCA (ICC = 0.45, [0.26–0.61]).
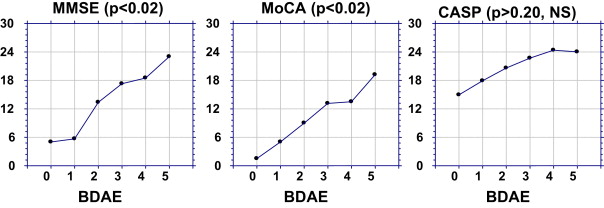
The BDAE score had a statistically significant impact on the total scores of the MMSE ( P < 0.02) and MoCA ( P < 0.02), since the most aphasic patients exhibited a poorer score ( Fig. 1 ). This impact was less pronounced for the CASP ( P > 0.20, NS). The most aphasic patients (BDAE at “0”) had a mean MMSE at 5/30, vs. 23/30 for the less aphasic ones (BDAE at “5”), amounting to an 18-point differential. This differential was 17.7 points for the MoCA vs. only 9.1 points for the CASP (score brought down to 30).
The mean administration time was significantly longer for the CASP (13 ± 4 min) than for the MMSE (8 ± 3 min, P < 10 −6 ). It was longer (not statistically significant) than for the MoCA (11 ± 5 min, P = 0.23).
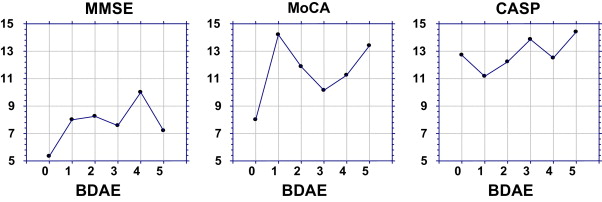
The BDAE score did not have a statistically significant impact on the time needed to administer the three batteries. However, we can note that the mean duration time for the administration of the MMSE and MoCA was shorter for patients with the most severe aphasia (BDAE score at “0”), compared to the mean times recorded for the other patients ( Fig. 2 ). On the other hand, the administration times were very homogenous for the CASP.
None of the items could be administered to all patients. For each scale the number of “impossible administrations” (at least one item could not be evaluated) was, as expected, higher in patients with a poor BDAE score ( Table 4 ). Most failures concerned patients with scores between 0 and 2.
| BDAE score | CASP | MMSE | MoCA |
|---|---|---|---|
| 0 (severe aphasia): 8 patients | 1 (13%) | 5 (63%) | 4 (50%) |
| 1: 9 patients | 3 (33%) | 6 (67%) | 4 (44%) |
| 2: 12 patients | 3 (25%) | 4 (33%) | 4 (33%) |
| 3: 8 patients | 1 (13%) | 1 (13%) | 1 (13%) |
| ≥ 4 (moderate aphasia): 7 patients | 0 (0%) | 0 (0%) | 0 (0%) |
| Total “non-achievable” | 8 | 16 | 13 |
For each scale, the three items that proved the most difficult to administer to patients were:
- •
CASP: item 7 “image recall” ( n = 5), item 5 “conflict resolution” ( n = 4) and item 6 “Line Bisection Test” ( n = 4);
- •
MMSE: item 3 “attention and calculation” ( n = 14), item 4 “recall” ( n = 11) and item 1 “orientation” ( n = 9);
- •
MoCA: item 6 “abstraction” ( n = 9), item 7 “recall” ( n = 9) and item 4 “attention” ( n = 7).
For the 5 patients for whom the item “image recall” from the CASP could not be administered, we quoted either “not possible”, or “0” for the recall items of the other two tests. Conversely, among the 11 patients who could not answer the “memory” item of the MMSE, 9 were able to answer the “image recall” item from the CASP, including 5 with a score at 4/6, 5/6 or 6/6. Regarding the MoCA: for the seven patients for whom the administration of the test was impossible, 5 of them answered the “recall image” item from the CASP, including two with a score at 6/6. As expected, these two patients had severe verbal expression disorders (BDAE at 0 or 1) but no comprehension impairments.
For the 9 patients for whom the “orientation” item of the MMSE was not possible, 8 were able to answer the “calendar” item of the CASP and 5 of them obtained a score of 4/6, 5/6 or 6/6 (generally, correct indication for at least the year and month). The same was true for the MoCA: among the 6 patients for whom that item was not possible, 5 answered the “calendar” item from the CASP, including 3 with a score of 5/6 or 6/6. Just like the “memory” item, these patients had severe verbal expression disorders (BDAE at 0 or 1), but without any comprehension impairments.
1.4
Discussion
We designed the CASP, a time-efficient evaluation scale for cognitive disorders post-stroke, adapted for administration to aphasic patients with verbal expression impairments and we compared it to the MMSE and MoCA in terms of feasibility in aphasic stroke patients.
In our series, none of the items from the three scales were achievable for all of these 44 patients and the administration of at least one item was impossible for 17 of them. This underlines the difficulty in evaluating cognitive functions in aphasic patients.
The CASP obtained the lowest rate of “impossible administration” because it does not require any verbal answer (except for the “verbal expression” item obviously). Furthermore, all the patients with severe verbal expression were able to complete it. The hazard of the study recruitment meant that only 3 patients fitted this description, including two with very severe impairments. This probably explains why the comparison of the percentage of “impossible administration” between the CASP and the MoCA was not statistically significant ( P = 0.21), whereas, the CASP-MMSE comparison was in fact statistically significant.
At the origin of our study, there were some items that drew our attention: memory and orientation items, not testable with the MMSE and MoCA in case of aphasia. In daily practice, teams are regularly confronted with the presence of these impairments in aphasic patients. It is in fact an important issue for physicians (and essential for families) in preparing the patient for being discharged home. We assumed that it would be possible to evaluate memory and orientation with the CASP in a large proportion of aphasic patients. This was the case in our study, especially in case of severe impairments predominant in the verbal expression. Furthermore, about half of the patients deemed “not testable” for these items by the MMSE and MoCA, obtained a good score to these corresponding items in the CASP. However the purely visual nature of the “recall image” test of the CASP did represent an issue for 3 patients who did however pass the corresponding items in the MMSE and MoCA. All three of them (G-14, G-19 and CCR-11) presented lesions of the posterior hemisphere and 2 of them (G-14 and CCR-11) exhibited clinical elements of cortical blindness. In reality, it is sometimes difficult for aphasic patients to differentiate test failure and failing to understand the instructions. However, patients G-14 and G-19 obtained a score of “0” on the memory items of the MMSE and MoCA. We can thus truly wonder if there was a real failure in passing the “image recall” item of the CASP (score at “0”) rather than the patients not having understood the instruction (“not achievable” item). For CCR-11, the visual gnostic disorders were much more severe than the aphasia (BDAE at 3) and there was no doubt regarding their impact on the result (item was really “not achievable”). This patient in fact obtained a score of “0” for the constructive praxis test of the MMSE and the graphic series from the CASP was quoted as “not achievable”.
In fact, it is quite certain that purely visual items, adapted to language disorders, were problematic in case of neurological visual impairments (NVI). This only concerned between one and 3 patients in our series of aphasic patients with left hemisphere stroke, but we should expect more failures (score at “0”) in right-sided stroke patients and as a consequence an overestimation of memory impairments. This in fact brings up the more general issue of the neuropsychological evaluation: just like aphasia interferes with verbal tests, NVI interfere with tests (memory or others) involving visual aids. With the CASP we can avoid the problem by helping the right-sided stroke patient (who will not be aphasic in the majority of cases) in recognizing the presented imaged and/or name the item by replacing the images by words expressed by the examiner, just like in the MMSE. More specifically, regarding the unilateral spatial neglect, which in PM&R departments (subacute phase) most often concerns the left hemisphere, our experience with the CASP underlined that organizing the images and words in columns and on the right side of the printed sheets made the tests administrable in the great majority of cases.
In this study, the three scores were highly correlated between themselves. This result was a priori expected, as showed by Aggarwal for the MMSE and MoCA in a PM&R setting . However, in Aggarwal’s article, only Pearson correlation coefficient ( r ) was presented (null hypothesis “H 0 : no correlation between both scores”), whereas no indication was given on the concordance between scores. In our study, we were expecting (and even hoping for) a poor concordance between the CASP and the other two tests. In fact, among aphasic patients, but nevertheless able to answer all items of the MMSE (in this case, n = 28) or the MoCA (in this case, n = 31), some encountered difficulties related to the strictly verbal nature of the questions and/or answers. Consequently, for these patients, aphasia might interfere with the other cognitive functions tested in the MMSE and MoCA and have a greater impact on their total score than for the CASP’ score. This, in fact, was clearly the case in our study. Furthermore, for any patient (aphasic or not), the structure difference between the three batteries does indeed induce a poor concordance between the scores. For example in the MMSE, the “orientation” item has twice more influence on the total score (10 points/30 = 1/3) than in the CASP (6 points/36 = 1/6). Similarly, the only visual item of the MMSE (reproduction of a drawing) has a lot less influence on the final score (1 point/30) than all the neurovisual items from the CASP (6 points/36). Another element, gestural praxis assessment is only available in the CASP. Overall, these three batteries do not:
- •
evaluate exactly the same functions;
- •
give them the same importance;
- •
measure them in the exact same manner.
It is thus natural for the concordance between their score to be poor. Contrarily to the choice made by the authors of the MMSE or MoCA we gave the same importance, i.e. 6 points/36, to the six cognitive functions evaluated and recommend to users to use the “profile” presentation of the score (“5/4/6/3/1/4”) in addition to the total score.
A short administration time is, by definition, essential for a battery of tests designed to “quickly” screen for cognitive disorders. The CASP administration was timed at 13 ± 4 min in average, which is very satisfactory. However this time was longer than for the MoCA, (11 ± 5 min, difference not statistically significant) and for the MMSE (8 ± 3 min, statistically significant).
It is logical that patients with “severe” aphasia obtain a poorer score to tests that evaluate, among other things, language capacities. However, the difference for the mean scores between the “more” aphasic and “less” aphasic patients was in our study twice as important for the MMSE and MoCA (18 and 17.7 points/30) than for the CASP (9.1 points, score brought down to 30). This suggests that the severity of language impairments negatively influenced the MMSE and MoCA scores, well beyond what could be explained by aphasia alone or associated cognitive impairments. Consequently, one might question the validity of certain items of the MMSE and MoCA in our study (under-evaluated performances?).
Finally, the fact that the mean administration times for the MMSE and MoCA were slightly weaker in patients with severe language impairments suggests also that these impairments might have influenced the administration of these tests. This was not the case for the CASP.
1.5
Conclusion
The analysis of some clinical aspects of aphasic patients is a recurring issue and some authors tried to bring a solution: strictly behavioral scale to evaluate depression , visual analog scale (VAS) for evaluating pain , simplification of the syntax for a quality of life questionnaire . In daily clinical practice, just like in the framework of clinical research, evaluating cognitive disorders such as memory or orientation impairments remains difficult in aphasic patients and restricted to experimented physicians, speech and language therapists or neuropsychologists. The CASP renders this evaluation accessible to “non-experts” such as young medical residents who are at the patient’s bedside and at the forefront of daily clinical practice. There is still a need to evaluate its feasibility in stroke patients with right hemisphere lesions, then validate it post-stroke (right and left hemisphere), this is actually ongoing in the framework of a French Clinical Research Program (PHRC) This PHRC will analyze the validity, reliability and responsiveness to change of the CASP, as well as setting norms according to age ranges. Furthermore, histoclinical correlation studies will be made possible by the systematic collection of data on the lesion location.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Funding: the CASPER study is supported by the French Clinical Research Program 2012.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







