Fatigue is a common and potentially debilitating symptom of amyotrophic lateral sclerosis (ALS). Questionnaire studies show that ALS subjects have increased subjective fatigue. Physiologic studies demonstrate that ALS subjects have increased physical fatigue, both central and peripheral in origin. No treatment has been proved effective through evidence-based medicine; however, modafinil (Provigil) may be a helpful pharmacologic treatment. Palliative care measures, such as noninvasive ventilation and high-frequency chest wall oscillation, may also reduce fatigue.
The hallmark of amyotrophic lateral sclerosis (ALS) is progressive muscular weakness that leads to immobility, loss of ability to speak and swallow, and eventual respiratory failure . Because there are no effective treatments for the disease and the only approved pharmacologic treatment (Riluzole) extends survival by only 2 months, the focus of ALS care is supportive care to palliate symptoms and improve quality of life. There are two primary examples of supportive care interventions in ALS. Percutaneous endoscopic gastrostomy (PEG) is used for administering tube feedings to prevent the negative effects of dehydration and starvation in people with ALS who are losing the ability to swallow . Noninvasive positive pressure ventilation (NIPPV), delivered via a mask strapped over the mouth and nose, is used to maintain oxygenation when respiratory muscle function is impaired. This intervention extends survival and improves quality of life through beneficial effects on sleep and emotional status .
Fatigue is an understudied clinical problem in ALS and is often overlooked by clinicians who care for people with ALS. About 20% of ALS patients indicate that they have severe suffering . Because it is a rapidly progressive and fatal disease without a cure , some patients with the option of physician-assisted suicide will choose to do so. In Oregon, where physician-assisted suicide is legal, ALS patients are 25 times more likely than those dying of other terminal illnesses to die by lethal ingestion . In the Netherlands, 20% of all people with ALS die by euthanasia or physician-assisted suicide . In addition, many ALS patients decline treatments that both sustain life and improve quality of life (QoL), such as noninvasive ventilation and PEG . It is possible that more people with ALS would accept life-sustaining treatments and be less interested in physician-assisted dying if symptoms, such as fatigue, were better addressed to improve quality of life. Thus, it is imperative for health care providers to understand the mechanisms of fatigue and treat fatigue effectively to improve quality of life in patients with ALS.
Approaching fatigue in ALS
One can evaluate fatigue systematically following the chart in Fig. 1 . The first step in assessing fatigue in ALS is to use a questionnaire to evaluate subjective fatigue. Questionnaires commonly used for fatigue include the Multidimensional Fatigue Inventory (MFI), the Fatigue Severity Scale (FSS), the Piper Fatigue Scale, and the Visual Analog Scale of fatigue. The author prefers to use the MFI because it is a multidimensional instrument that evaluates physical fatigue and mental fatigue independently. After one establishes that subjective physical fatigue is present, one can use a continuous supramaximal force exercise paradigm or an intermittent submaximal force exercise paradigm to quantify physical fatigue objectively in a laboratory setting. While questionnaires assess the severity of subjective fatigue over days to weeks, exercise protocols assess the severity of physical fatigue over seconds to minutes. Therefore, the severity of subjective fatigue as measured by questionnaires may not correlate with the severity of physical fatigue measured by exercise protocols.
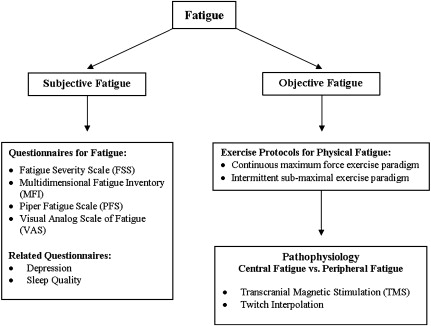
To further understand the pathophysiology of physical fatigue, one can use additional techniques, such as twitch interpolation and transcranial magnetic stimulation, to identify whether the fatigue is peripheral (in the neuromuscular junction or the muscles) or central (in upper motor neurons and lower motor neurons).
Though research into fatigue in ALS has been limited, a number of studies have scratched the surface by employing each of these techniques. However, it is not clear if fatigue in ALS: (a) remains an independent symptom that is treatable after treating other symptoms, such as depression, pain, or dyspnea; (b) simply reflects the effects of other pathophysiologic constructs, such as sleep problems, dyspnea, or depression; or (c) is associated with other symptoms because they share a common cause .
ALS subjects report more subjective fatigue than normal controls
The author has used the MFI to compare the fatigue in ALS subjects with normal controls and examined the influence of fatigue on QoL, as measured by the McGill Quality of Life (MQoL) questionnaire . The MFI is a 20-item self-report instrument designed to measure fatigue . The 20 items cover five dimensions of fatigue: general fatigue, physical fatigue, mental fatigue, reduced motivation, and reduced activity. This study demonstrated that subjects with ALS reported more fatigue than normal controls, and fatigue severity correlated inversely with QoL. Compared with normal controls, ALS subjects reported more general fatigue, physical fatigue, and reduced activity, but not mental fatigue or reduced motivation ( Fig. 2 ). Most importantly, the author found that the severity of fatigue and depression did not correlate with disease severity, as measured by the ALS functional rating scale (ALSFRS), muscle strength, or disease duration. Severity of general fatigue contributed significantly to more severe physical symptoms, as measured by a subscale in the MQoL. Higher depression scores were correlated with increased mental fatigue but not physical fatigue. This cross-sectional pilot study demonstrated that fatigue and depression in ALS subjects are important predictors of QoL and that fatigue and depression are independent of physical function. Although the author and colleagues still do not have an effective treatment for ALS, treating fatigue and depression aggressively in ALS patients may improve their QoL.
In addition to muscle weakness, depression, poor pulmonary function, and poor quality of sleep might contribute to fatigue in ALS patients. Depression is common in ALS patients: more than 40% of ALS subjects in two studies had depressive symptoms . Approximately 10% of ALS subjects met criteria for major depressive disorder . The author and colleagues need further study to examine if treating depression aggressively will reduce fatigue in ALS patients.
Poor pulmonary function might cause fatigue through mechanisms such as insomnia, hypersomnia, and exercise intolerance. Standard pulmonary function testing has been used to assess the progression of respiratory muscle weakness in ALS. Forced vital capacity (FVC) is indicative of both inspiratory and expiratory muscle strength. Peak inspiratory pressures are more specific indicators of diaphragmatic function, which correlates with a subject’s ability to ventilate, and are inversely correlated with the frequency and degree of sleep-disordered breathing. Lower peak inspiratory pressures are associated with lower QoL in ALS patients .
Poor quality of sleep and excessive daytime somnolence might also contribute to fatigue in ALS. ALS patients have reduced total sleep time, reduced sleep efficiency, and increased apneas and hypopneas . Whether specific aspects of sleep affect fatigue in ALS patients remains unknown.
ALS subjects have more objective fatigue than normal controls
Physical fatigue in ALS cannot be totally attributed to muscle weakness. Sanjak and colleagues examined the relationship between weakness and fatigue in 54 ALS subjects compared with normal controls. Subjects performed 30 seconds of sustained maximal voluntary isometric contraction of elbow flexors, knee extensors, and ankle dorsiflexors, using a computerized force measurement system and standardized testing procedures. They used a continuous maximum force exercise paradigm to calculate the Fatigue Index ( Fig. 3 B). Fatigue was greater in ALS subjects than in normal controls (mean = 23% versus 15%, p <.001) in all muscles, including muscles that were not clearly weak. They found that muscle weakness and fatigue were poorly correlated in ALS subjects.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





