Most clinical guidelines do not recommend routine use of epidural steroid injections for the management of chronic low back pain. However, many clinicians do not adhere to these guidelines. This comprehensive evidence overview concluded that off-label epidural steroid injections provide small short-term but not long- term leg-pain relief and improvement in function; injection of steroids is no more effective than injection of local anesthetics alone; post-procedural complications are uncommon, but the risk of contamination and serious infections is very high. The evidence does not support routine use of off-label epidural steroid injections in adults with benign radicular lumbosacral pain.
Key points
- •
A systematic review of the literature suggests that off-label epidural steroid injections provide short-term but not long-term (>12 weeks) relief of leg pain and improvement in function in patients with benign lumbosacral radicular syndrome. The clinical importance of steroid benefits is small (<10 points improvement on a 100-point scale).
- •
Different steroids are similarly effective in reducing pain and disability in the short term but do not do so in a dose-responsive manner.
- •
Injection of steroids is no more effective than injection of local anesthetics alone.
- •
Postprocedural complications are uncommon, but the risk of contamination and serious infections is very high.
- •
Evidence is insufficient to posit an association between short-term effectiveness of steroid injections and differing patient characteristics.
- •
Based on high-quality evidence, routine use of off-label epidural steroid injections in adults with benign radicular lumbosacral pain is not recommended.
Introduction
The prevalence of acute and chronic low back pain in adults has increased more than 100% in the last decade and continues to increase dramatically in the aging population, affecting both men and women in all ethnic groups. Low back pain contributes to lost productivity, disability, and substantial health care expenditures, and is the leading determinant of years lived with disability.
The goals of conservative treatment of chronic low back pain (pain persisting for >12 weeks) are to decrease pain, improve function, reduce opioid use, and obviate spinal surgery. Most available clinical guidelines do not recommend routine use of invasive treatments, including epidural steroid injections, for the management of chronic low back pain.
However, many clinicians do not adhere to these guidelines. The Office of Inspector General (OIG) of the United States Department of Health and Human Services concluded that more than 30% of epidural injections were inappropriate, and resulted in $45 million of improper Medicare payments, and an additional $23 million in improper facility payments. Lack of adherence to guideline recommendations among generalist physicians may relate to difficulties in communicating to patients the benefits and harms of available treatments for low back pain.
Many studies, including systematic reviews, suffer from inconsistent methodology and provide conflicting conclusions and recommendations about the benefits and harms of epidural treatments, making clinical decisions even more difficult. Individual randomized studies use various definitions of low back pain, evaluate different steroid administration routes and doses, and provide inconsistent measures of treatment success. Therefore, this article aims to provide a comprehensive overview of currently available reviews and primary epidemiologic studies to inform evidence-based clinical decision making in the treatment of benign chronic low back pain.
Introduction
The prevalence of acute and chronic low back pain in adults has increased more than 100% in the last decade and continues to increase dramatically in the aging population, affecting both men and women in all ethnic groups. Low back pain contributes to lost productivity, disability, and substantial health care expenditures, and is the leading determinant of years lived with disability.
The goals of conservative treatment of chronic low back pain (pain persisting for >12 weeks) are to decrease pain, improve function, reduce opioid use, and obviate spinal surgery. Most available clinical guidelines do not recommend routine use of invasive treatments, including epidural steroid injections, for the management of chronic low back pain.
However, many clinicians do not adhere to these guidelines. The Office of Inspector General (OIG) of the United States Department of Health and Human Services concluded that more than 30% of epidural injections were inappropriate, and resulted in $45 million of improper Medicare payments, and an additional $23 million in improper facility payments. Lack of adherence to guideline recommendations among generalist physicians may relate to difficulties in communicating to patients the benefits and harms of available treatments for low back pain.
Many studies, including systematic reviews, suffer from inconsistent methodology and provide conflicting conclusions and recommendations about the benefits and harms of epidural treatments, making clinical decisions even more difficult. Individual randomized studies use various definitions of low back pain, evaluate different steroid administration routes and doses, and provide inconsistent measures of treatment success. Therefore, this article aims to provide a comprehensive overview of currently available reviews and primary epidemiologic studies to inform evidence-based clinical decision making in the treatment of benign chronic low back pain.
Methods
The authors formulated the following clinical questions as the basis for this overview. (1) What are the short-term and the long-term efficacy and safety of epidural steroid injections in the treatment of chronic radicular lumbosacral pain in community-dwelling adults? (2) What patient characteristics may modify treatment benefits and harms?
The target population was defined as community-dwelling adults age 18 and older with benign radicular lumbosacral pain lasting more than 12 weeks. Lumbosacral radicular syndrome was defined as radiculopathy, nerve root compromise, nerve root compression, disc herniation, radiculitis, nerve root pain, or nerve root entrapment. This review relied on diagnostic methods provided by the authors of the original studies. It was determined whether the following factors modified the effects of treatment: patient age, gender, ethnicity, socioeconomic status, duration of pain, and prior response to analgesics; and comorbidities including obesity, osteoporosis, and history of spinal trauma, diabetes, or arterial hypertension.
Eligible interventions included off-label epidural steroid injections administered with or without fluoroscopic guidance. The effects of different routes of steroid administration (eg, caudal, transforaminal, or interlaminar) and different steroid formulations and doses ( Appendix 1 Tables 1 and 2 ; available at www.pmr.theclinics.com ) were examined as well as the frequency of injections if provided by the investigators. Comparators included placebo, epidural injection of anesthetics, and nonpharmacologic treatments including physical therapy or acupuncture/acupressure.
Outcomes included pain, global symptom relief, functional improvement and reduction in disability, patient perception of improvement, return to work, use of opioid and nonopioid analgesia, need for surgery, and quality of life. Outcomes at both short-term and long-term (>12 weeks) follow-up were examined. Minimum clinically important improvement in outcomes was defined as a greater than 50% reduction in pain or disability scores.
Also examined were the adverse effects of treatment related to epidural technique (eg, dural puncture, hematoma or infections) and those related to the pharmacologic effects of the injected drugs (eg, weight gain, fluid retention, hyperglycemia or hypertension).
Study Inclusion Criteria
Guidelines, systematic reviews, and randomized controlled clinical trials (RCTs) in English, and large observational cohorts to assess treatment safety, were examined. Harms were defined as the totality of all possible adverse consequences of an intervention. Investigators sometimes defined harmful effects as unrelated to steroid treatments. Harms were analyzed regardless of how investigators related them to treatments.
Study Exclusion Criteria
Studies of pregnant women and patients with recent trauma, tumors, or cauda equina syndrome were excluded, as were studies of children and nursing home residents, studies of surgical treatments, and studies that did not examine the effect of epidural steroid injections on eligible patient outcomes.
The authors relied on the study design used for indexing of the references in bibliographic databases ( Appendix 2 ; available at www.pmr.theclincs.com ). However, clinical reviews that did not meet systematic review definitions were excluded regardless of indexing.
Search Strategy
In accordance with a protocol developed a priori (registration number CRD42014007011), all relevant articles published in English up to January 10, 2014, in PubMed, Embase, Science Direct, and the Cochrane Library (see Appendix 2 for the exact search strings) were identified. To identify unpublished data, a search was made of the trial registry clinicaltrials.gov . The bibliographies of identified articles were scanned, and study investigators were contacted for additional publications. A separate search for relevant cost-effectiveness studies was conducted.
Two authors performed initial eligibility determination for the studies, and all co-authors contributed to resolving differences. Information was abstracted about study population, interventions, comparators, and outcomes. Minimum datasets (eg, number of the subjects in treatment groups and events) were abstracted to estimate absolute risk difference, relative risk, and number needed to treat for categorical variables. Means and standard deviations of continuous variables were abstracted to calculate standardized mean differences, odds ratios, and numbers needed to treat, assuming a 25% control group rate of improvement in pain or function. Two co-authors cross-checked abstracted data with the texts of the original articles.
Quality Assessment of the Studies
This overview used the Assessment of Multiple Systematic Reviews (AMSTAR) scale for systematic reviews, the Appraisal of Guidelines for Research and Evaluation (AGREE II) scale for clinical guidelines, the Cochrane risk of bias tool for randomized trials, and the risk of bias tool for nonrandomized studies from the Agency for Healthcare Research and Quality. A low risk of bias was assumed when RCTs met all the risk of bias criteria, a medium risk of bias if at least 1 of the risk-of-bias criteria was not met, and a high risk of bias if 2 or more risk-of-bias criteria were not met. An unknown risk of bias was assigned for the studies with poorly reported risk-of-bias criteria.
Synthesis of Evidence
An overview of the reviews was conducted following the framework of the Cochrane collaboration. Although no meta-analysis was performed, the authors calculated absolute risk difference, number needed to treat, and the number of attributable events per 1000 treated based on data from the published randomized trials, using Meta-Analyst software and STATA software. Statistical significance was evaluated at a 95% confidence level. Correction coefficients for zero events were used as a default option in both software programs, and intention to treat was used for evidence synthesis. Superiority of drugs under comparison was hypothesized. This review relied on pooling criteria and heterogeneity statistics from published meta-analyses, and assessed reporting bias following the recommendations of the Agency for Healthcare Research and Quality.
To examine the role of patient characteristics, a search was undertaken for subgroup analyses by patient demographics, pain type, prior treatment response, and comorbidities in systematic reviews and randomized trials, including significant interaction effects.
Quality Assessment of the Body of Evidence
The authors assigned quality of evidence ratings as high, moderate, low, or very low, according to risk of bias in the body of evidence, directness of comparisons, precision and consistency in treatment effects, and the evidence of reporting bias, using GRADE methodology.
Treatment effect estimates were defined as precise when pooled estimates had reasonably narrow 95% confidence intervals and pooled sample size was greater than 300. Justification of the sample size was not included in grading of the evidence, nor were post hoc statistical power analyses conducted.
In assessing the quality of evidence in all studies, the authors looked for a dose-response association, the strength of association, and evidence of any reporting bias. The strength of the association was evaluated, defining a priori a large effect when the relative risk was greater than 2 and a very large effect when the relative risk was greater than 5. A small treatment effect was construed when the relative risk was significant but less than 2. For standardized continuous measures of pain and function, the magnitude of the effect was defined as small, moderate, and large, corresponding to standardized mean differences in standard deviation units of 0 to 0.5, 0.5 to 0.8, and greater than 0.8, respectively.
A high quality of evidence was assigned to well-designed RCTs with consistent findings. The quality of evidence was downgraded to moderate if at least 1 of 4 strength-of-evidence criteria was not met; for example, moderate quality of evidence was assigned if there was a medium risk of bias in the body of evidence or if the results were not consistent or precise. The quality of evidence was downgraded to low if 2 or more criteria were not met.
A low quality of evidence was assigned to nonrandomized studies, and upgraded for the rating if there was a strong or dose-response association. Evidence was defined as insufficient when no studies provided valid information about treatment effects. This approach was applied regardless of whether the results were statistically significant.
Strength of Recommendations
Strength of the recommendations was assigned based on overall quality of evidence, balances between benefits and harms, and cost, using GRADE methodology.
Results
A total of 344 references were retrieved, which included 79 references for this review ( Fig. 1 ). Eighteen systematic reviews were identified that synthesized data from 65 RCTs ( Appendix 1 Table 3 ). Eleven publications of RCTs that were omitted from the reviews or published after the reviews were identified ( Appendix 1 Table 4 ). Excluded studies are referenced in Appendix 2 to assure transparency in studies selection. There was evidence of reporting bias, because only 3 of 7 eligible studies registered in clinicaltrials.gov had been published. The reporting quality of the primary studies was generally poor, with unclear allocation concealment and incomplete reporting of treatment-effect means with variance that precluded comprehensive meta-analyses of data pertaining to all primary and secondary outcomes.
Benefits of Epidural Steroids
No consistent evidence was found for clinically important sustained benefits from epidural steroids in adults with radicular lumbosacral pain. Primary studies and systematic reviews did not necessarily relate outcomes to exact duration of symptoms or to a success from prior treatments.
Systematic reviews provided conflicting conclusions. The high-quality systematic review by members of the Cochrane Collaboration did not distinguish among interlaminar, caudal, or transforaminal epidural injection techniques for lumbosacral radicular syndrome, and found no clinically important benefits with use of epidural steroids (see Appendix 1 Table 3 ).
By contrast, the reviews authored by members of the American Society of Interventional Pain Physicians (ASIPP) included results from both RCTs and observational studies stratified by injection techniques and type of spinal disorders, and concluded that there is good evidence of short-term and long-term pain reduction and improvement in function with epidural steroids (see Appendix 1 Table 3 ). These reviews categorized results from individual studies as positive, negative, or statistically insignificant, but failed to address the clinical importance of these categories and did not provide rates of clinically significant improvements in pain and disability, number needed to treat, or attributable events for clinical decision making. The primary studies and the reviews reported multiple outcomes at different short-term and long-term time points without considering statistical multiple hypothesis testing.
The reviews conducted by evidence-based practice centers concluded that there are short-term but not long-term benefits with epidural steroids in patients with sciatica (see Appendix 1 Table 3 ). The authors of one recent high-quality review converted continuous measurements of pain and disability to common scales from 0 (no pain or disability) to 100 (worst possible pain or disability), and reported weighted means of short-term pain reduction of 6% and disability of 3%. These small statistically significant effects have questionable clinical importance.
Injection Technique
No single specific injection technique improved back pain. A statistically significant short-term reduction in leg pain was reported with caudal injection, but not with interlaminar or transforaminal approaches. A statistically significant reduction in long-term leg pain was reported with transforaminal injection, but not with caudal or interlaminar approaches. A statistically significant reduction in short-term disability was reported with caudal injection, but not with interlaminar or transforaminal approaches.
Steroid Dose
Systematic reviews found no evidence to suggest that a series of epidural injections was any more effective than a single injection (see Appendix 1 Table 3 ). Individual RCTs found no evidence of improvement in steroid benefits with increasing dose (see Appendix 1 Table 4 ).
Comparative Effectiveness of Epidural Steroids
Individual RCTs found no consistent evidence of superior efficacy of one steroid over the others (see Appendix 1 Table 4 ). Moreover, injection of anesthetic alone resulted in reduction in pain and disability similar to that derived from a combination of steroids with anesthetic (see Appendix 1 Table 4 ).
Epidural Steroid Safety
Harms associated with epidural steroid injections were rarely reported in individual RCTs and systematic reviews ( Appendix 1 Table 5 ). One large observational study of more than 10,000 epidural injections reported that crude rates of selected postsurgical complications were rare (see Appendix 1 Table 5 ). Other reviews reported a rate of dural puncture frequency between 2% and 5%, and rare cases of postdural puncture syndrome. One recent systematic review analyzed reports from the Centers for Disease Control and Prevention and the Food and Drug Administration as well as all published evidence of contaminated epidural steroid injections, and concluded that these substantial harms outweighed any short-term benefits. Several case series published during the last decade reported that development of abscesses after epidural steroids sometimes resulted in paraplegia and death. Primary studies including RCTs and observational studies did not measure all well-known steroid-related adverse effects ( Appendix 1 Table 6 ).
Cost-Effectiveness
Conclusions about cost-effectiveness of epidural steroid injections are inconsistent. A recent analysis concluded that caudal epidural steroid injections are cost-effective when compared with combined conservative and invasive treatments.
Quality of the Evidence
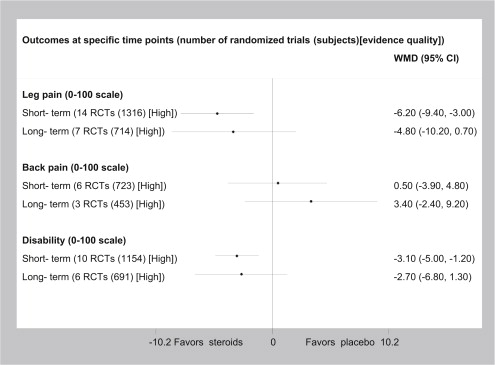
High-quality evidence suggests that epidural steroid injections provide short-term but not long-term leg-pain relief and improvement in function for patients with lumbosacral radicular syndrome when compared with placebo ( Fig. 2 ). The clinical importance of these small changes in pain and disability is questionable. High-quality evidence also suggests that caudal corticosteroid injections are better than placebo in reducing leg pain at short-term but not long-term follow-up ( Appendix 1 Table 7 , Tables 1–4 ). Low-quality evidence suggests that caudal corticosteroid injections result in short-term improvement in disability. Very low-quality evidence suggests that transforaminal corticosteroid injections are better than placebo in reducing leg pain at long-term follow-up, with no improvement in disability (see Appendix 1 Table 7 , Tables 1–4 ). Low-quality evidence supports similar effectiveness of different steroids on pain and disability. Low-quality evidence finds no dose-response association between steroid doses and improvement in outcomes.
| Outcome | Type of Pain a | Effect Measure (95% CI) NNT | OR b (95% CI) | No. of RCTs (Subjects) Quality of Evidence | Comments |
|---|---|---|---|---|---|
| Intervention: Epidural Corticosteroid Injection vs Placebo (All Techniques Combined) | |||||
| Outcome: Back pain at short-term follow-up | Sciatica c | WMD 0.5 (−3.9 to 4.8); NNT = NS | OR 0.8–1.2 (NS) | 6 RCTs (723) High | No difference |
| Outcome: Back pain at long-term follow-up | Sciatica | WMD 3.4 (−2.4 to 9.2); NNT = NS | OR 1.1 (NS) | 3 RCTs (453) High | No difference |
| Outcome: Leg pain at short-term follow-up | Sciatica | WMD −6.2 (−9.4 to −3.0); NNT = 10 | OR 0.5 (0.4; 0.8) | 14 RCTs (1316) High | Favors epidural corticosteroid injection |
| Outcome: Leg pain at long-term follow-up | Sciatica | WMD −4.8 (−10.2 to 0.7); NNT = NS | OR 0.6 (0.3; 1.0) | 7 RCTs (714) High | No difference |
| Intervention: Epidural Caudal Corticosteroid Injection vs Placebo | |||||
| Outcome: Leg pain at short-term follow-up | Sciatica | SMD −0.32 (−0.61; −0.04); NNT = 11 | OR 0.6 (0.3; 0.9) | 2 RCTs (192) High | Favors epidural corticosteroid injection |
| Outcome: Leg pain at long-term follow-up | Sciatica | SMD −0.30 (−0.58; −0.01); NNT = 12 | OR 0.6 (0.3; 1.0) | 2 RCTs (187) Moderate | No difference |
| Intervention: Epidural Interlaminar Corticosteroid Injection vs Placebo | |||||
| Outcome: Leg pain at short-term follow-up | Sciatica | SMD −0.40 (−0.80; 0.00); NNT = 9 | OR 0.5 (0.2; 1.0) | 6 RCTs (613) Moderate | No difference |
| Outcome: Leg pain at long-term follow-up | Sciatica | SMD −0.16 (−0.71; 0.39); NNT = 20 | OR 0.7 (0.3; 2.0) | 2 RCTs (298) High | No difference |
| Intervention: Epidural Transforaminal Corticosteroid Injection vs Placebo | |||||
| Outcome: Leg pain at short-term follow-up | Sciatica | SMD −0.24 (−0.52; 0.05); NNT = 14 | OR 0.6 (0.4; 1.1) | 3 RCTs (270) Moderate | No difference |
| Outcome: Leg pain at long-term follow-up | Sciatica | SMD −0.93 (−1.52; −0.33); NNT = 5 | OR 0.2 (0.1; 0.6) | 1 RCT (48) Very low | Favors epidural transforaminal corticosteroid injection |
| Intervention: Epidural Corticosteroid Injections (All Techniques Combined) vs Control | |||||
| Outcome: Any pain at 12 mo | Low back pain | SMD −0.12 (−0.27; 0.04); NNT = 27 | 0.8 (0.6; 1.1) | 9 RCTs (683) Moderate | No difference |
a In this table and hereafter, patient subpopulations were defined according to the definitions provided in the studies.
b In this table and hereafter, ORs were calculated based on WMD.
c Radiculopathy, nerve root compromise, nerve root compression, lumbosacral radicular syndrome, disc herniation, radiculitis, nerve root pain, and nerve root entrapment.
| Outcomes | Type of Pain | Effect Measure (95% CI) NNT | OR (95% CI) | No of RCTs (Subjects) Quality of Evidence | Comments |
|---|---|---|---|---|---|
| Intervention: Epidural Corticosteroid Injection vs Placebo (All Techniques Combined) | |||||
| Outcome: Disability at short-term at follow-up | Sciatica a | WMD −3.1 (−5.0 to −1.2); NNT = 16 | OR 0.7 (0.5; 0.9) | 10 RCTs (1154) High | Favors epidural corticosteroid injection |
| Outcome: Disability at long-term at follow-up | Sciatica | WMD −2.7 (−6.8 to 1.3); NNT = NS | OR 0.7 (0.4; 1.1) | 6 RCTs (691) High | No difference |
| Intervention: Epidural Caudal Corticosteroid Injection vs Placebo | |||||
| Outcome: Disability at short-term at follow-up | Sciatica | SMD −0.31 (−0.60; −0.03); NNT = 11 | OR 0.6 (0.3; 0.9) | 2 RCTs (192) Low | Favors epidural caudal corticosteroid injection |
| Intervention: Epidural Interlaminar Corticosteroid Injection vs Placebo | |||||
| Outcome: Disability at short-term at follow-up | Sciatica | SMD −0.19 (−0.39; 0.02); NNT = 17 | OR 0.7 (0.5; 1.0) | 3 RCTs (383) Low | No difference |
| Intervention: Epidural Transforaminal Corticosteroid Injection vs Placebo | |||||
| Outcome: Disability at short-term at follow-up | Sciatica | SMD −0.12 (−0.39; 0.16); NNT = NS | OR 0.8 (0.5; 1.3) | 2 RCTs (205) Low | No difference |
| Intervention: Epidural Corticosteroid Injections (All Techniques Combined) vs Control | |||||
| Outcome: Disability at 12 mo | Low back pain | SMD 0.09 (−0.35; 0.54); NNT = NS | 1.2 (0.5; 2.6) | 9 RCTs (843) Moderate | No difference |
a Radiculopathy, nerve root compromise, nerve root compression, lumbosacral radicular syndrome, disc herniation, radiculitis, nerve root pain, and nerve root entrapment.
| Comparisons | Type of Pain | ARD (95% CI) NNT | Relative Risk (95% CI) | No. of RCTs (Subjects) Evidence Quality | Comments |
|---|---|---|---|---|---|
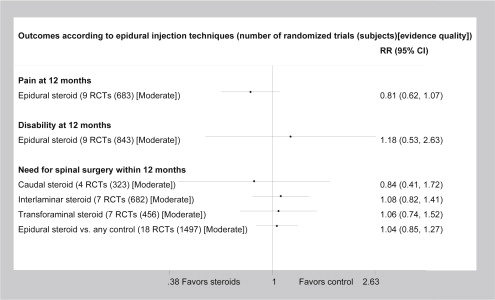
| Caudal steroids vs control | Low back pain | 0.00 (−0.07; 0.07) NNT = NS | 0.8 (0.4; 1.7) | 4 RCTs (323) Moderate | No differences |
| Interlaminar steroids vs control | Low back pain | 0.01 (−0.05; 0.07) NNT = NS | 1.1 (0.8; 1.4) | 7 RCTs (682) Moderate | |
| Transforaminal steroids vs control | Low back pain | 0.00 (−0.08; 0.07) NNT = NS | 1.1 (0.7; 1.5) | 7 RCTs (456) Moderate | |
| Epidural steroids vs control | Low back pain | 0.00 (−0.03; 0.04) NNT = NS | 1.0 (0.9; 1.3) | 18 RCTs (1497) Moderate |
| Harm Definitions | Incidence of Harms | Relative Incidence Rate | Studies | Evidence Quality | Comments |
|---|---|---|---|---|---|
| Intra- and short-term postsurgical complications | No differences | No differences | 5 RCTs (429) | Low | No difference |
| Serious adverse effects from contaminated epidural steroids injections | >14,000 patients were exposed to contaminated steroids, >337 developed meningitis, and 25 died of aspergillosis | Substantial increase in risk of serious harms associated with epidural steroids injections | Systematic review, 43 references to the CDC and FDA reports and observational studies | High | Epidural steroid injections were associated with substantial risk of infectious contaminations and patient harms |
| Epidural abscess | Death and paraplegia were reported in patients with abscesses after epidural steroid injections | No control | 11 noncontrolled case series and case reports | Very low | Multiple cases of abscesses after epidural steroid injections were published in the last several decades |
Moderate-quality evidence suggests that epidural steroids are not better than anesthetics in improving pain or disability or in reducing the need for surgery ( Fig. 3 ). High-quality evidence indicates that short-term postprocedural complications are uncommon but that the risks of contamination and serious infections are very high (see Appendix 1 Table 5 , Table 4 ).
Evidence of an association between patient characteristics and steroid effects was insufficient.
Discussion
This review found no evidence of sustained, clinically important improvement in back pain or disability, and no reduction in need for surgery or opioid use after epidural steroid injections in adults with chronic low back pain. Current evidence suggests that epidural steroid injections provide some short-term improvement in radicular lumbosacral pain and disability. This conclusion is based on statistically significant differences that are likely not clinically significant in most treated patients. These results are consistent with recommendations in most published guidelines on the treatment of radicular low back pain. Most of these guidelines do not mention epidural steroids or recommend them ( Appendix 1 Table 8 ). Those that do comment on the uneven quality of evidence relating to the 3 injection techniques (caudal, interlaminar, and transforaminal approaches), and provide different recommendations for symptoms of differing etiology including disc herniation and/or radiculitis, discogenic pain without disc herniation, and spinal stenosis. Guidelines from the ASIPP provide a stronger recommendation in favor of epidural steroid use; however, the guideline development process did not require transparent peer review by all societies concerned with treatment of adults with radicular lumbosacral pain. The British Pain Society guideline recommends that only patients with severe radicular pain lasting more than 2 weeks should be referred to a specialist who can consider magnetic resonance imaging–guided steroid injections or surgery.
The North America Spine Society guideline recommends fluoroscopy-guided epidural steroid injections for short-term symptom relief in patients with neurogenic claudication or radiculopathy. The American College of Physicians and the American Pain Society concluded that epidural steroids are potential treatment options in cases of prolapsed lumbar disc with persistent radicular symptoms not responding to noninvasive therapy.
Individualized treatment recommendations in the guidelines are largely based on expert opinion, as no well-designed RCTs demonstrate sustained clinically important benefits from epidural steroids for specific patient populations. For informing individualized treatment decisions, the evidence is insufficient in clarifying differences in steroid effects by patient characteristics including demographics, causes of back pain, and comorbidities. Evidence is also insufficient to conclude that epidural steroids have any salutary effect on patients’ quality of life.
Limitations of this work include the fact that the authors relied on published evidence and did not contact principal investigators for additional information about study methodology and unreported outcomes. Treatment effects from published randomized controlled trials and one meta-analysis were recalculated. Suboptimal quality of reporting in primary studies precluded comprehensive meta-analysis of all examined outcomes.
Implications for Future Research
Future research should combine patient-level data from all published and unpublished randomized trials, categorize treatment effect by clinical importance, and provide the number needed to treat to explore clinically meaningful improvement in patient subpopulations by demographics, prior treatment response, spinal pathology, and comorbidities. Patient registries would provide additional insight into long-term safety and in to the association between quality of care rendered by health care providers and meaningful patient outcomes.
Implications for Clinical Practice
In concordance with some current guidelines, clinicians perform epidural injections in increasing numbers of patients. Patient preferences for choosing invasive treatments for low back pain remain unclear. Based on this analysis, the authors conclude that the evidence does not support routine use of epidural steroid injections for chronic radicular lumbosacral pain. Patients should be informed that benefit from steroid injection is likely to be short-lived and may carry the risk of contamination. If chosen for treatment, epidural injections should be administered in specialized centers by trained physicians, prioritizing patient safety and taking all precautions to eliminate the risk of contamination. However, whenever possible, guideline-recommended conservative treatment options should be offered first in adults with chronic low back pain.
Appendix 1: Epidural Injections for Radicular Lumbosacral Pain: A Systematic Review
| Brand Name | |||||
|---|---|---|---|---|---|
| Depo-Medrol | Depo-Medrol | Kenalog | Celestone | Decadron | |
| Formulation | Methylprednisolone | Methylprednisolone | Triamcinolone acetonide | Betamethasone preservative-free | Dexamethasone sodium phosphate |
| Amount of steroid (mg/mL) | 40 | 80 | 40 | 6 | 4 |
| Polyethylene glycol | 29.1 | 28.2 | |||
| Polysorbate 80 | 1.94 | 1.88 | 0.4 | ||
| Monobasic sodium phosphate | 6.8 | 6.59 | 3.4 | ||
| Benzyl alcohol | 9.16 | 8.8 | |||
| Dibasic sodium phosphate | 9 | ||||
| Edetate disodium | 7.1 | ||||
| Benzalkonium chloride | 0.1 | ||||
| Sodium sulfite | 0.2 | 1 mg | |||
| Drug | Equivalent Dose (mg) | Epidural Dose (mg) | Anti-Inflammatory Potency | Sodium Retention Capacity | Duration of Adrenal Suppression | ||
|---|---|---|---|---|---|---|---|
| Intramuscular | Single Epidural | Three Epidurals | |||||
| Hydrocortisone | 20 | N/A | 1 | 1 | N/A | N/A | N/A |
| Depo-Methylprednisolone (Depo-Medrol) | 4 | 40–80 | 5 | 0.5 | 1–6 wk | 1–3 wk | N/A |
| Triamcinolone acetonide (Kenalog) | 4 | 40–80 | 5 | 0 | 2–6 wk | N/A | 2–3 mo |
| Betamethasone (Celestone Soluspan) | 0.6 | 6–12 | 33 | 0 | 1–2 wk | N/A | N/A |
| Dexamethasone (Decadron) | 0.75 | 8–16 | 27 | 1 | N/A | N/A | N/A |
| Reference (Sorted by Publication Year) Quality | Design | Patients Intervention vs Control | Outcomes | Results |
|---|---|---|---|---|
| Systematic reviews | ||||
| Choi et al, 2013 Assessment of Multiple Systematic Reviews (AMSTAR) = 73% | Review of 29 RCTs | 843 adults with low back pain Epidural steroid injections vs epidural control | Pain relief, functional improvement in 6–12 mo and need for surgery | No benefits of epidural steroid injections for low back pain at 6–12 mo follow-up. Epidural steroids did not significantly decrease the number of patients who underwent subsequent surgery |
| Bicket et al, 2013 AMSTAR = 64% | Review of 43 RCT | 3641 adults with back or neck pain with or without radiculopathy. Epidural corticosteroids injection vs epidural injection of another analgesic or nonepidural injection (intramuscular or ligamentous) | Positive response as >50% relief of pain and pain score reduction on an 11-point rating scale at 12 wk after the treatments | Positive response after epidural steroids vs epidural nonsteroid injection (n = 2 RCTs) ARD = 0.04 (95% CI −0.01 to 0.10) Pain reduction mean difference −0.27 (95% CI −0.51 to −0.04) Epidural steroids vs nonepidural injection (n = 7 RCTs) ARD = 0.31 (0.20–0.42) Pain reduction mean difference −0.12 (95% CI −0.44 to 0.21) |
| Bui & Bogduk, 2013 AMSTAR = 37% | Review of 19 nonrandomized studies of CT-guided epidural steroids | Number of patients not specified Adults with radicular pain ongoing CT-guided, lumbar transforaminal injection of steroids vs fluoroscopy guided vs standard protocol injections | Relief of pain; the radiation exposure and complications associated with the procedure, the drug, and the radiation | Success rates (defined as >50% relief of pain between 1 and 6 mo after treatment) 34%–62% Radiation exposure with fluoroscopy is 3–5 times less than after low-dose CT protocol and 18–40 times less than a standard protocol. Case reports suggested that CT guidance did not eliminate spinal cord injury or spinal cord infarction |
| Epstein, 2013 AMSTAR = 40% | Review of 43 observational studies | Number of patients with epidural steroid injections for acute vs chronic low back pain not specified | Adverse effects related to epidural procedure and steroids | The multitude of risks attributed to epidural steroid injections outweighs the benefits including transient pain relief. Recent reports from the FDA, CDC, and published studies demonstrated that 14,000 patients were exposed to contaminated steroids, 337 developed meningitis, and 25 died of aspergillosis. The patients experienced spinal fluid leaks (0.4%–6%), positional headaches (28%), adhesive arachnoiditis (6%–16%), hydrocephalus, air pulmonary embolism, urinary retention, allergic reactions, intravascular injections (7.9%–11.6%), stroke, blindness, neurologic deficits/paralysis, hematomas, and seizures |
| Pinto et al, 2012 AMSTAR = 80% | Review of 22 RCTs | 2184 patients with sciatica ongoing epidural corticosteroid injections vs placebo | Pain intensity, disability Oswestry Disability Index (ODI) scores and Roland-Morris Questionnaire scores converted to the same 0–100 scale | Meta-analysis demonstrated that epidural corticosteroid injections for sciatica compared with placebo decreased leg pain in the short term (mean difference, 6.2 [95% CI, 9.4–3.0]) and disability in the short term (mean difference, 3.1 [95% CI, 5.0–1.2]). The long-term (>12 mo) pooled effects were smaller and not statistically significant Approximately one-half of the included trials (13 of 23) involved patients with a mixed duration of symptoms acute, subacute, or chronic). Treatment effects did not differ by symptom duration |
| Parr et al, 2012 AMSTAR = 80% | Review of 11 RCTs and 5 nonrandomized studies | 1252 patients with chronic low back pain ongoing caudal epidural steroid vs nonsteroid injections | Pain relief, improvement in functional status, psychological status, return to work, and reduction in opioid intake; complications | Qualitative review concluded that for lumbar disc herniation with radiculitis, the evidence was good for short-term and long-term (>6 mo) relief with local anesthetics with steroids (3 of 4 RCTs). For discogenic or axial pain without disc herniation, radiculitis, facet joint pain, or sacroiliac joint pain, the evidence was fair for caudal epidural injections (1 RCT). For spinal stenosis, available evidence was fair (1 long-term RCT) for local anesthetic with or without steroids. The common complications included infection, either local or epidural, abscess, discitis; hematoma formation, spinal cord infarction; extraepidural placement with subcutaneous injection; subdural injection, dural puncture with postlumbar puncture headache, nerve damage, intracranial air injection or increased intracranial pressure; pulmonary embolism; and adverse effects of steroids |
| Manchikanti et al, 2012 AMSTAR = 80% | Review of 13 RCTs and 10 nonrandomized studies | 2363 patients with chronic lumbar spinal pain undergoing lumbar transforaminal epidural steroid injections vs nonsteroid epidural injections | Pain relief, improvement in functional status, psychological status, return to work, and reduction in opioid intake; complications | Qualitative review concluded that at short- and long-term follow-up for radiculitis secondary to disc herniation, the evidence was good for local anesthetics and steroid. For radiculitis secondary to spinal stenosis, the evidence was fair for steroids. For axial pain, the evidence was insufficient. The most common complications were related to neural trauma, vascular trauma, intravascular injection, and infection. None of the studies included in an effectiveness analysis showed any major complications |
| Benyamin et al, 2012 AMSTAR = 80% | Review of 15 RCTs and 11 nonrandomized studies | 3001 patients with chronic low back and lower extremity pain undergoing lumbar interlaminar epidural injections vs nonsteroid epidural injections | Pain relief, improvement in functional status, psychological status, return to work, and reduction in opioid intake; complications | Qualitative review concluded that for radiculitis secondary to disc herniation, the evidence was good for treatments with local anesthetics and steroids. For radiculitis secondary to spinal stenosis, the evidence was fair for treatments with local anesthetic or steroids. For axial pain without disc herniation, the evidence is fair for treatments with fluoroscopically guided epidural steroid injections |
| Benoist et al, 2012 AMSTAR = 40% | 21 systematic reviews | Number of patients and symptom duration not specified. Patients with low back pain with radiculopathy undergoing epidural steroid injections vs nonsteroid epidural injections | Pain and function, complications | Qualitative review of published reviews concluded that Cochrane high-quality systematic reviews combining interlaminar, caudal, or foraminal approaches for several pathologic conditions, such as lumbosacral radicular syndrome, did not show any benefits with steroid injections. Other systematic reviews found a moderate short-term benefit of epidural steroid injections vs placebo in patients with disc herniation and radiculitis. Dural puncture frequency rate was between 2% and 5%; rare cases of postdural puncture syndrome including headache, nausea, and vertigo, and cases of paraplegia have been reported |
| Quraishi, 2012 AMSTAR = 40% | Review of 5 RCTs | 368 patients undergoing lumbar transforaminal or periradicular infiltration of glucocorticoids for chronic radicular pain vs nonsteroid epidural injections | Visual Analogue Score (VAS) and ODI | Transforaminal epidural injection of steroids did not reduce pain (standardized mean difference in VAS 0.2 in favor of steroids; 95% CI: −0.41 to 0.00) or disability (standardized mean difference in ODI 0; 95% CI −0.21 to 0.20). One study following patients to 12 mo did not find any significant difference in VAS and ODI between treatment and control groups |
| Lewis et al, 2011 AMSTAR = 80% | Review of 12 RCTs | Number of patients not specified. Patients with sciatica ongoing epidural steroid injections vs nonsteroid epidural injections. Most studies included patients with both acute and chronic sciatica | Global effect on pain and function | Mixed treatment comparisons meta-analysis demonstrated that odds ratio of global effect with epidural injection was 3.1 (95% CI 1.8–5.5). Epidural corticosteroid injections were cost-effective for sciatica Epidural injections (WMD −12.9 [95% CI −20.91 to −5.14]) resulted in better pain relief when compared with inactive controls. When compared with usual care, epidural injections (OR 3.8) resulted in better overall improvement The results are not reported by the exact time of follow-up |
| Jordan et al, 2010 AMSTAR = 50% | 5 systematic reviews and 5 RCTs | Number of patients by symptom duration not specified. Patients with herniated lumbar disc ongoing epidural steroid injections vs nonsteroid epidural injections | Pain, disability | The published studies reported inconsistent results. Evidence was insufficient to draw conclusions on effects of epidural injections of corticosteroids for both outcomes and at short- and long-term follow-up |
| Dagenais et al, 2010 AMSTAR = 80% | Review of 10 guidelines on low back pain treatments | 8 European general practice guidelines and 2 guidelines from USA. Six discussed chronic low back pain and 6 discussed low back pain with neurologic involvement | Substantial neurologic improvement | Among 10 guidelines only 1 guideline from the Belgian Health Care Knowledge Center recommended epidural steroid injections |
| Henschke et al, 2010 AMSTAR = 64% | Review of 2 RCTs | 88 adults with chronic (>12 wk duration) low back pain (including subjects with radiculopathy or any other nonspecific degenerative pathology, such as osteoarthritis). Interventions: epidural injection with corticosteroid vs nonsteroid epidural injections or targeted steroid placement with a spinal endoscope | Pain relief | Only very low-quality evidence that epidural corticosteroid injections did not reduce pain over short term when compared with benzodiazepine injection or targeted steroid placement with a spinal endoscope |
| Roberts et al, 2009 AMSTAR = 70% | Review of 9 RCTs | Number of patients not specified. Patients with radicular pain ongoing lumbosacral transforaminal epidural steroid injections vs nonsteroid epidural injections | Pain, function | Lumbosacral transforaminal epidural steroid injections were more effective than placebo in treating radicular symptoms (fair evidence). Lumbosacral transforaminal epidural steroid injections were more effective than interlaminar and caudal epidural injections for radicular pain (good evidence) supporting their use in the treatment of lumbosacral radicular pain. In patients with subacute or chronic radicular symptoms, a single lumbosacral transforaminal epidural steroid injection had similar efficacy on short- and long-term pain and function as a single transforaminal injection |
| Rabinovitch et al, 2009 AMSTAR = 80% | Review of 14 RCTs and 1 controlled trial | 886 patients with radicular leg pain and/or low back pain ongoing lumbar epidural injection vs nonsteroid epidural injections; exact symptom duration not specified | Pain relief as immediate (<6 wk); short-term (6 wk–3 mo); intermediate (>3 mo–1 y); and long-term (>1 y) | There were statistically significant correlations between the amounts of fluid injected epidurally and pain relief in patients with radicular leg pain and/or low back pain immediately after the injection |
| Staal et al, 2008 AMSTAR = 90% | Review of 7 RCTs | 101 patients with low back pain with symptoms persisting for at least 1 mo contributed to the analysis Epidural injection vs nonsteroid epidural injections | Pain relief, general improvement in function | There was insufficient evidence to support the use of epidural steroid injections for subacute and chronic low back pain and improvement in function. Statistical pooling was not possible because of clinical heterogeneity in the trials |
| Novak & Nemeth, 2008 AMSTAR = 60% | Review of 11 RCTs, 1 prospective controlled trial, and 2 prospective cohort studies | Number of patients by symptom duration not specified Studies examined repeat epidural injections for radicular pain secondary to herniated nucleus pulposus or spinal stenosis vs nonsteroid epidural injections | Pain, function | Evidence was insufficient to support series of epidural injections instead a single injection. Statistical pooling was not conducted |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







