Entrapment Neuropathies and Other Focal Neuropathies (Including Carpal Tunnel Syndrome)
Lawrence R. Robinson
General Approaches to the Study of Entrapment
There are a number of general considerations when evaluating any patient with suspected entrapment or focal neuropathy. In this chapter, approaches to the history and physical examination, timing of electrodiagnostic changes, pathophysiology, and principles of localization will be discussed, as well as initial electrodiagnostic approaches.
History
A directed history is critical to performing the electrodiagnostic medical consultation. It is required for generating an appropriate list of differential diagnoses and planning the electrophysiologic examination. Moreover, many times the electrodiagnostic medical consultant has more experience in the diagnosis of focal neuropathies and other neuromuscular conditions than the patient’s referring physician and thus may think of alternative diagnoses that the referring physician had not considered.
There are a number of specific components to the history especially pertinent to the evaluation. The quality and precise distribution of symptoms, combined with an intimate knowledge of peripheral nervous system anatomy, will usually be very helpful in developing a sensible differential diagnosis. Finding out whether symptoms are intermittent or constant will suggest a likelihood of finding abnormalities on the electrophysiologic examination; constant symptoms are more likely to be associated with electrophysiologic abnormalities than intermittent symptoms. While symptoms of entrapment neuropathies are usually initially reported in one or two limbs, one should also ask about other limbs to rule out a more generalized process. The patient presenting with hand numbness, for example, could have entrapment neuropathy in the upper limbs; however, if the feet are also involved, then one might perform a wider search for a peripheral polyneuropathy. When patients report the sudden onset of symptoms upon awakening, this should prompt extensive questioning about where and how the patient slept and if he or she was intoxicated or otherwise medicated. If there is a recent history of surgery or trauma, a detailed history may help point to which areas of the peripheral nervous system might have been placed at risk.
While an extensive search of the past medical history is not always productive in the evaluation for possible entrapment neuropathies, there are several questions that should always be asked. One should routinely ask for the medications the patient is taking. This brings up not only other prior pertinent diagnoses that may not have been mentioned (e.g., diabetes mellitus), but also possible
exposure to neurotoxic agents or anticoagulants. One should elicit any history of systemic disease that might contribute to the chief complaint, such as a history of diabetes mellitus, extensive alcohol intake, or rheumatologic disease. Also, one should know whether the presenting symptoms have occurred in the past so that one is prepared for the electrophysiologic findings of prior events. A history of prior trauma may be pertinent, as in the case of tardy ulnar palsy where an old elbow injury predisposes to a later ulnar neuropathy. The patient’s vocational history may play a role in diagnosis, as many occupations increase the risk of developing focal entrapment neuropathies.
exposure to neurotoxic agents or anticoagulants. One should elicit any history of systemic disease that might contribute to the chief complaint, such as a history of diabetes mellitus, extensive alcohol intake, or rheumatologic disease. Also, one should know whether the presenting symptoms have occurred in the past so that one is prepared for the electrophysiologic findings of prior events. A history of prior trauma may be pertinent, as in the case of tardy ulnar palsy where an old elbow injury predisposes to a later ulnar neuropathy. The patient’s vocational history may play a role in diagnosis, as many occupations increase the risk of developing focal entrapment neuropathies.
The family history becomes pertinent when one is considering congenital diseases. Peripheral polyneuropathies and myopathies are not uncommonly inherited, and it can be very revealing to find a family history of similar neurologic symptoms. There is an autosomally dominant condition known as familial predisposition to pressure palsies (1) that may be relevant when considering entrapment neuropathies.
Physical Examination
While the history often contributes most significantly to establishing a differential diagnosis, a directed physical examination is useful in providing more objective evidence of focal peripheral nervous system dysfunction. In most cases, the four most important components of the examination are muscle strength, sensation, muscle stretch reflexes, and provocative signs. The strength examination should be directed to all four limbs, both to look for widespread abnormalities as well as to assess any underlying poor effort. Although in some cases weakness is severe, in most referrals the weakness is mild or subtle. Optimally, muscles should be tested at or near their “break” points as opposed to the large range where resistance cannot be overcome. Thus, one must be sure to obtain a maximum mechanical advantage in performing the muscle strength testing. Useful techniques include applying force as far as possible from the joint to obtain a maximal lever arm, putting particularly strong muscles at added stretch to put them at a mechanical disadvantage, and using gravity and body weight as an aid to maximally stress antigravity muscles. Simply testing dorsiflexion or plantarflexion at the ankle against manual resistance, for example, is insufficient to fully stress the dorsiflexors or plantarflexors. It is preferable to have the patient walk on his heels and on his toes or perform 10 toe rises on each foot.
Sensory testing should be directed at eliciting subtle deficits in sensation. As opposed to the patient with spinal cord injury, where one is looking for a very gross sensory level, patients with entrapment neuropathies often have mild or difficult-to-elicit sensory losses. Simply finding out whether the patient can distinguish pinprick from dull touch is usually insufficient for all but the most severe deficits. One should compare pinprick and light touch sensations in a suspected area with an asymptomatic area, such as the cheek, or with the other side if it is not symptomatic. A useful technique is to touch first the asymptomatic area and then the symptomatic area, asking the patient, “If the feeling you have in this (asymptomatic) area is 100%, how much is it in this (the symptomatic area)?” Two-point discrimination often detects milder deficits in sensation that are missed with simply testing pinprick.
Muscle stretch reflexes are probably the most objective finding in examination of the peripheral nervous system, in that they are not easily influenced by patient cooperation or reporting (though they are by the level of relaxation). Reflexes are normal in many focal entrapment neuropathies that are at distal sites, but they will help to rule out more proximal lesions.
There are several useful provocative tests that can be used in the physical examination prior to electrophysiologic studies. Phalen’s test is a moderately sensitive and specific test for detecting median nerve compression at the wrist. This test is performed by keeping the wrist in sustained flexion for 60 seconds and monitoring for reproduction of paresthesias. Tinel sign, which was originally developed for detecting the most distal site of peripheral nerve regeneration, is sensitive but not very specific. It can be elicited over the median nerve at the wrist or ulnar nerve at the elbow in the case of entrapment, but many asymptomatic control subjects also have a positive test over unaffected peripheral nerves. The Flick sign is elicited by asking the patient what he or she does when awakened at night by symptoms. A Flick sign is present when the patient “flicks” the wrist in
response to this question. None of the aforementioned signs is particularly sensitive or specific when applied to those patients referred to the electrodiagnostic laboratory (2).
response to this question. None of the aforementioned signs is particularly sensitive or specific when applied to those patients referred to the electrodiagnostic laboratory (2).
Timing
Before embarking upon the electrodiagnostic examination, it is critical to appreciate the timing since the onset of symptoms, particularly when there is trauma or the sudden onset of symptoms. False-negative or misleading conclusions can result from not understanding the influence of timing since the onset of symptoms. Demyelination and marked axon loss produce electrophysiologic changes immediately if one can stimulate proximal and distal to the lesion. More proximal lesions do not immediately produce changes on distal nerve conduction studies or needle electromyography (EMG) of resting muscle. Whether the lesion is proximal or distal, distinction between demyelination and axon loss cannot be made until after the time for Wallerian degeneration has passed. These concepts, as applied to traumatic neuropathies, are covered further in other sources (see Chapter 3) (3).
Day 1 after a Lesion
Immediately after onset of a demyelinating or axon loss lesion, electrophysiologic changes may be subtle. On needle EMG the only potential abnormality may be a change in motor unit potential (MUP) recruitment, with reduced or discrete recruitment in severe lesions. Mild lesions will not produce noticeable changes in recruitment. Nerve conduction studies distal to the site of the lesion will not be changed, but stimulation proximal to a lesion with distal recording may produce a small-amplitude or absent response. Otherwise, nerve conduction studies and EMG will be unremarkable at day 1.
Days 7 to 10
Seven days after a complete nerve lesion, Wallerian degeneration will have progressed to the point where distal stimulation of motor axons elicits no motor response. Ten days after onset of a complete lesion, sensory nerve action potentials (SNAPs) will be absent as well (3). Incomplete lesions will produce less marked changes, roughly in proportion to the number of axons lost, but with similar timing. Therefore, 7 to 10 days after onset, nerve conduction studies can distinguish a neurapraxic injury (in which distal amplitudes will be normal) from an axon loss lesion (in which distal amplitudes will be reduced or absent).
Days 14 to 21
Two to three weeks after onset of injury, the needle EMG starts to show fibrillation potentials and positive sharp waves. Proximal muscles (those nearest the site of injury) demonstrate these abnormalities earlier and distal muscles later. Fibrillations and positive sharp waves may be persistent for several months or even many years after a single injury, depending on the extent of reinnervation. Fibrillation amplitudes are sometimes helpful in determining the chronology of the lesions; fibrillation potentials larger than 100 μV indicate a lesion probably less than 1 year old (4).
Reinnervation
The timing and types of electrophysiologic changes consequent to reinnervation will depend in part upon the mechanism of reinnervation. When reinnervation is a result of axonal regrowth from the site of the lesion, such as in complete axonal injuries, the appearance of new MUPs will not occur until motor axons have had sufficient time to regenerate the distance between the lesion site and the muscle (usually proceeding at roughly 1 mm per day or 1 inch per month). When these new axons first reach the muscle, they will innervate only a few muscle fibers, producing short-duration, small-amplitude potentials, formerly referred to as nascent potentials. With time, as more muscle fibers join the motor unit, the MUPs will become larger, more polyphasic, and longer in duration.
MUP changes will also develop when reinnervation occurs by axonal sprouting from intact axons. Polyphasicity and increased duration develop as newly formed, poorly myelinated sprouts supply the recently denervated muscle fibers. As the sprouts mature, large-amplitude, long-duration MUPs develop and usually persist indefinitely.
Pathophysiology
Whenever possible, provide the referring physician some indication of the pathophysiology of the peripheral nerve lesion (e.g., neurapraxia,
demyelination, or axon loss). Neurapraxia is best demonstrated, assuming at least 7 days have passed, when there is focal conduction block on nerve conduction studies with a large-amplitude compound muscle action potential (CMAP) or SNAP elicited distal to the site of the lesion and a smaller or absent response with more proximal stimulation. There is some debate about whether purely neurapraxic injuries have fibrillation potentials or positive sharp waves on needle EMG. Some report that positive sharp waves can exist in purely neurapraxic lesions, while others point out the difficulty in demonstrating that the lesion is purely neurapraxic with zero axon loss.
demyelination, or axon loss). Neurapraxia is best demonstrated, assuming at least 7 days have passed, when there is focal conduction block on nerve conduction studies with a large-amplitude compound muscle action potential (CMAP) or SNAP elicited distal to the site of the lesion and a smaller or absent response with more proximal stimulation. There is some debate about whether purely neurapraxic injuries have fibrillation potentials or positive sharp waves on needle EMG. Some report that positive sharp waves can exist in purely neurapraxic lesions, while others point out the difficulty in demonstrating that the lesion is purely neurapraxic with zero axon loss.
Axon loss lesions are usually demonstrated by evidence of denervation on needle EMG examination as well as small-amplitude CMAP and SNAP responses with stimulation and recording distal to the site of the lesion. While needle EMG is a more sensitive indicator for the presence of any motor axon loss, measurement of the distal CMAP or SNAP amplitude is a better quantitative measure of the degree of axon loss and of prognosis. There is often a mixture of axon loss and neurapraxia in focal neuropathies.
Demyelination is best demonstrated by slowing of conduction, often with conduction block. Slowing of conduction may take the form of slowed conduction velocities, prolonged distal latencies, and increased temporal dispersion. Slowing of conduction does not always mean that demyelination has occurred; axon loss, particularly of the faster-conducting fibers, will produce mild slowing of conduction as well.
Estimating Prognosis
Prognosis of a peripheral nerve lesion is related to the pathophysiologic process that has occurred, the degree of axon loss, the time since onset, and the distance between the lesion and the target muscles. Lesions that have had extensive axon loss are less likely to have full recovery of function. Unfortunately, electrophysiologic measures cannot assess the integrity of supporting structures around the nerve and hence cannot distinguish axonotmesis from neurotmesis. Neurotmesis, which has marked disruption of supporting structures, carries a much worse prognosis for regeneration than axonotmesis, in which the supporting structures are largely intact. In these cases, careful periodic EMG re-examination of proximal muscles (those expected to reinnervate first) will give the best information as to the ultimate prognosis for full reinnervation.
Lesions that are predominantly neurapraxic have a much better prognosis; conduction block in these lesions rarely lasts more than 2 to 3 months. Demyelinated lesions also have a better prognosis than axon-loss lesions, but the specific prognosis will depend upon what intervention is taken, such as release of entrapment sites.
When axon loss is present, there is a critical window of 18 to 24 months for peripheral nerve regeneration to occur before the target muscles cannot be reinnervated any longer (3). Since peripheral nerves regenerate roughly 1 inch per month, proximal lesions with a great deal of axon loss have a poorer chance of reinnervating distal hand or foot muscles. Even for proximal muscles, surgical intervention, if needed, must allow enough time for axons to grow to the muscle while reinnervation can still occur (3).
Principles of Localization
There are a number of principles useful for localizing peripheral nerve lesions based upon the electrophysiologic examination. Conventionally, in primarily axonal lesions or in proximal lesions where one cannot stimulate both proximal and distal to an entrapment site, needle EMG is often used to diagnose and localize abnormalities. Knowing the branch points along the nerve, one can examine the muscles supplied by each branch and infer lesion localization based upon the point at which the muscles change from normal to abnormal. Thus, localization is based upon finding abnormalities distal to a branch point with normal findings in proximal muscles.
This approach, however, sometimes provides an erroneous site. Sir Sidney Sunderland (5) has shown that fascicles within peripheral nerves intertwine considerably as they move proximally through the limbs. Fascicles supplying the flexor carpi ulnaris muscle, for example, are not uniquely placed proximally within the ulnar nerve as it joins the medial cord of the brachial plexus. However, fascicles do become organized within peripheral nerves several centimeters prior
to branch points and, in this example, fascicles destined to supply the flexor carpi ulnaris become organized within the ulnar nerve several centimeters prior to exiting the nerve to supply the muscle. Consequently, even though ulnar nerve entrapment at the elbow usually occurs proximal to the branch to the flexor carpi ulnaris, this muscle is usually spared in ulnar neuropathy at the elbow, as the fascicles for this muscle are isolated in a relatively protected area of the nerve at the entrapment site. If one were basing localization only on needle EMG using the known branch points, one would erroneously place these lesions distal to the branch point and in the forearm.
to branch points and, in this example, fascicles destined to supply the flexor carpi ulnaris become organized within the ulnar nerve several centimeters prior to exiting the nerve to supply the muscle. Consequently, even though ulnar nerve entrapment at the elbow usually occurs proximal to the branch to the flexor carpi ulnaris, this muscle is usually spared in ulnar neuropathy at the elbow, as the fascicles for this muscle are isolated in a relatively protected area of the nerve at the entrapment site. If one were basing localization only on needle EMG using the known branch points, one would erroneously place these lesions distal to the branch point and in the forearm.
The ulnar nerve is not unique with regard to the intraneural topography, causing potential problems in localization. There have been cases reported of fibular (the peroneal nerve is now called the fibular nerve) neuropathy occurring proximal to the popliteal fossa but resulting in only deep fibular nerve lesions clinically (6). Sciatic neuropathies, even occurring near the hip joint, can result in predominantly common fibular nerve deficits. The fascicular structure within the fibular division of the sciatic nerve may make it more predisposed to injury than the tibial division (5). Thus, while EMG does make use of known anatomic branch points to arrive at localization, the electromyographer should be aware of the intraneural topography within the nerve and should remember that a partial lesion can be misleading.
Nerve conduction studies are best at localizing the site of pathology when there is demyelination. Focal slowing and conduction block, when present, can precisely localize a nerve lesion. A problem arises with localizing lesions based on nerve conduction studies when there is predominantly axon loss and little demyelination. In these cases conduction velocity throughout the nerve is mildly slowed due to loss of the faster-conducting fibers, but it is not focally or markedly slowed. While there is a diffuse reduction in CMAP or SNAP amplitude at all sites of stimulation (due to axon loss and subsequent Wallerian degeneration), there will be no focal drop in amplitude as one goes across the lesion site. Conduction block, in which there is a drop in amplitude of the CMAP as stimulation occurs distal and proximal to the lesion, is related only to demyelination and neurapraxia and will not be present in axon-loss lesions once Wallerian degeneration has occurred (about 7 days after onset).
Carpal Tunnel Syndrome
CASE 1
A 52-year-old woman reports a 6-month history of right-hand numbness and pain. This involves the whole hand, and she does not differentiate whether this is just palmar or also dorsal numbness. She also reports hand weakness, easily dropping small items. The pain and numbness often awaken her at night. She shakes her hand when awakened by these symptoms. She denies numbness in her feet. She also denies any neck or proximal upper limb pain. Past medical history is significant only for hypothyroidism and obesity. She works in a chicken-processing plant.
Physical examination is remarkable for normal strength proximally in the limb, though there is mild weakness of thumb abduction on the right. Sensation is reduced on the palmar surface of the right thumb, index finger, and long finger. Reflexes are 2+ and symmetric. Phalen’s sign and Tinel signs are present.
Differential Diagnosis
When presented with a patient with hand numbness, carpal tunnel syndrome (CTS) should usually be in the differential diagnosis. However, one should also consider the possibility of more diffuse or more proximal peripheral nervous system lesions.
When considering diffuse processes such as peripheral polyneuropathy, one should ask about symptoms in other limbs. If patients have symptoms such as numbness or tingling in the feet, one should consider polyneuropathy and look for risk factors in the medical history. Cervical spondylitic myelopathy could also present with hand and foot symptoms but should also have myelopathic features such as bowel or bladder dysfunction and hyperactive reflexes.
With diffuse hand numbness (i.e., more than just the median nerve distribution), one might consider multiple nerve lesions, such as both median and ulnar nerves. However, it is common for CTS
to present with diffuse hand numbness, or with numbness in the median and ulnar distributions (7). The differential diagnosis may also include more proximal median neuropathies, such as pronator syndrome, ligament of Struthers, flexor digitorum sublimis arch, and others. All such patients should be examined for weakness in more proximal limb muscles, and not just in the distal median nerve distribution. Brachial plexopathy and cervical radiculopathy should also be considered in the differential diagnosis. These would be more strongly considered if the patient has proximal limb symptoms or signs, neck pain, or other predisposing factors.
to present with diffuse hand numbness, or with numbness in the median and ulnar distributions (7). The differential diagnosis may also include more proximal median neuropathies, such as pronator syndrome, ligament of Struthers, flexor digitorum sublimis arch, and others. All such patients should be examined for weakness in more proximal limb muscles, and not just in the distal median nerve distribution. Brachial plexopathy and cervical radiculopathy should also be considered in the differential diagnosis. These would be more strongly considered if the patient has proximal limb symptoms or signs, neck pain, or other predisposing factors.
Clinical Presentation
Patients with CTS typically present with hand numbness and tingling. As noted above, the symptoms often extend outside the median nerve sensory distribution and can even involve the whole hand in a glove-type distribution (7). Symptoms are worse at night, and nocturnal awakening is common. Often when patients are awakened by their symptoms they shake or “flick” their hands. Some investigators report this Flick sign (patients flick their hand when asked what they do at night) as highly sensitive and specific (8), whereas others have found it to be of limited utility (2). Driving and hand use are also precipitating factors for symptoms.
There are a number of risk factors for CTS. Medical risk factors include hypothyroidism, obesity, rheumatoid arthritis, osteoarthritis, prior wrist fracture, diabetes, and pregnancy (9). Occupations that put individuals at risk for CTS are those that involve high-force or high-repetition hand movements. Medium- and light-duty industries commonly have workers with CTS.
The physical examination does not always show marked abnormalities in CTS. Strength testing may show weakness or atrophy of thenar muscles. When testing thenar muscles, one should be careful to separate these muscles from the long radial-innervated thumb extensors. The inexperienced examiner will sometimes test thumb extension rather than thumb abduction (the latter is perpendicular from the plane of the palm). Sensation may be reduced in the median distribution to pinprick or two-point discrimination. Muscle stretch reflexes are usually normal. Phalen’s test is performed by flexing the wrist for 1 minute. It is positive if this produces paresthesia in the median nerve distribution, reproducing the symptoms. The Tinel sign was originally developed for detecting nerve regeneration after traumatic neuropathies, but it is frequently applied to peripheral nerve entrapments such as CTS. This involves tapping over the nerve at the site of compression; a positive sign is paresthesia in the median nerve distribution, reproducing the symptoms. There is debate about the sensitivity and specificity of Phalen’s sign and the Tinel sign for detecting CTS. A recent review has suggested that they are of limited utility and of less value than thumb abduction strength or distribution of symptoms (10).
Optimal Strategies for Electrodiagnosis of Carpal Tunnel Syndrome
Although CTS is the most frequently seen entrapment neuropathy in the electrodiagnostic laboratory, multiple and varied approaches have been described for diagnosing this condition. The lack of uniformity in approach suggests that there are likely many unanswered questions on how to best diagnose patients with this condition (or, alternatively, many faculty seeking promotion and tenure!). Readers are encouraged to read a comprehensive review of methods for diagnosing CTS (11).
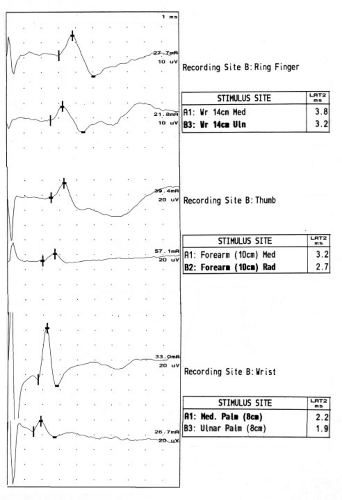
The general approach to nerve conduction studies should include measurement of sensory and motor conduction in the median nerve across the wrist, with comparison to nearby nerves that do not traverse the carpal tunnel. Conduction in sensory axons is usually affected before motor axons, though rarely motor axons are preferentially affected, possibly due to focal compression of the recurrent branch of the median nerve.
While there are many approaches to studying the median sensory nerve across the wrist, I rely upon three sensory nerve conduction studies (Fig. 10-1) that have literature support for a high degree of sensitivity and reasonable specificity in CTS (Table 10-1). These are comparison of the distal latency of the median and ulnar sensory antidromic conduction to the ring finger at 14 cm (ringdiff), comparison of the distal latency of the median and radial sensory antidromic conduction to the thumb at 10 cm (thumbdiff), and comparison of the distal
latency of the median and ulnar orthodromic conduction across the wrist with palmar stimulation at 8 cm (palmdiff) (11,12). Reference values can be easily remembered as “3, 4, 5”: the midpalmar studies are 0.3 ms or less, the ring finger 0.4 ms or less, and the thumb studies 0.5 ms or less. Values exceeding these reference values are suggestive of CTS.
latency of the median and ulnar orthodromic conduction across the wrist with palmar stimulation at 8 cm (palmdiff) (11,12). Reference values can be easily remembered as “3, 4, 5”: the midpalmar studies are 0.3 ms or less, the ring finger 0.4 ms or less, and the thumb studies 0.5 ms or less. Values exceeding these reference values are suggestive of CTS.
Temperature is well known to slow nerve conduction latencies and velocities. Thus, obtaining a median sensory latency will be dependent upon the temperature to a large degree. Much of this dependency, however, can be reduced by using comparisons of two nerves within the same limb (i.e., using the methods suggested above). Thus, to avoid influences of temperature as well as age, height, and other influential variables, it is preferable to use comparisons of median nerve latency to other nerves within the same hand rather than comparing the median sensory latency against a fixed standard reference value.
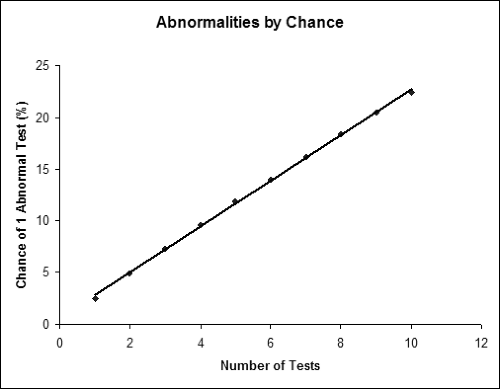
Given the number and variety of electrodiagnostic tests available for diagnosing CTS, the question that inevitably comes up is how many tests should be performed in each patient. At first, it is tempting to perform many tests so that one is more likely to detect any problem that might be subtle or might not be shown on a smaller number of tests. Also, by performing more tests, one could argue that one is making a more thorough assessment of different nerve fascicles than one could with a single test. There are, however, problems with performing multiple tests. The most significant problem is that of multiple comparisons. As one performs more and more tests, each of which has a 2.5% false-positive rate (under ideal circumstances), the chance of any one of the tests being abnormal goes up almost additively. Thus, performing two tests yields a 4.9% chance of at least one test being abnormal in a control population. For three tests, the false-positive rate is 7.3%; Figure 10-2 shows the false-positive rate as one performs greater numbers of tests. The false-positive rate is lower if one requires multiple (two or more) tests to be abnormal to make a diagnosis, but this will lower sensitivity. Another practical problem with performing more testing is simply the time and cost required to complete the study. Obviously one would not want to perform tests that do not add clinical or diagnostic information.
Thus, the question of how many tests to perform is not a trivial matter. The strategies for performing multiple tests should be decided upon before studying the patient and not handled in a casual manner. To address the question of how to interpret multiple tests, Robinson et al (13) have compared strategies of analyzing the three different distal sensory latency comparisons described above for CTS. This strategy is to summate the results from three tests into a single number. The combined sensory index is calculated as the sum of the three latency differences:
Combined sensory index = palmdiff + ringdiff + thumbdiff
Table 10-1 Nerve Conduction Results for Case 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This brings in the advantages of multiple tests (e.g., assessing multiple areas of nerve, enhancing reproducibility of findings) but does not create the problem of multiple comparisons. Thus, as long as one does three tests but only “looks at” the summated result from all three tests, one does not run into the problem of an additive false-positive rate. Using this approach, sensitivity is improved, with specificity still remaining high at 95%. These results assume a reference value for the combined sensory index of 0.9 ms or less. Thus, use of the combined sensory index has advantages of improved sensitivity and high specificity compared to doing multiple individual tests or even a single individual test. While this represents an improvement over single tests, it has also been noted that it might not be necessary to perform all three tests for the combined sensory index when one or more are extreme values (14).
Median motor conduction is an essential component of the electrodiagnostic evaluation of CTS. Not only will this allow the examiner to detect motor slowing across the wrist, but it will also detect the small number of individuals with CTS limited to motor slowing. Most commonly, studies are performed with recording over the abductor pollicis brevis (APB) and stimulation at the wrist (8 cm proximally) and the elbow. Some authors have also advocated stimulation in the palm to look for conduction block (neurapraxia) at the wrist; conceptually a much larger-amplitude response at the palm would suggest focal compression (15). However,
other authors (16) have reported that a substantial number of asymptomatic individuals have amplitude changes across the carpal tunnel that would suggest the presence of CTS. Due to stimulation of the deep ulnar nerve (which on average is 1.2 cm from the recurrent median nerve) (16) and/or crossing anomalous fibers from median to ulnar nerves, 53% of healthy control subjects have a palm/wrist difference in amplitude greater than reported control values, and 25% of control subjects have an amplitude ratio (palm/wrist) outside the reference range. Nevertheless, clinicians should consider stimulation of sensory nerves both proximal and distal to the site of compression (i.e., at the wrist and palm) to look for evidence of conduction block. This technique is less problematic than motor nerve stimulation in the palm, since it is unlikely that ulnar sensory responses will be volume-conducted to ring electrodes over the median innervated digits.
other authors (16) have reported that a substantial number of asymptomatic individuals have amplitude changes across the carpal tunnel that would suggest the presence of CTS. Due to stimulation of the deep ulnar nerve (which on average is 1.2 cm from the recurrent median nerve) (16) and/or crossing anomalous fibers from median to ulnar nerves, 53% of healthy control subjects have a palm/wrist difference in amplitude greater than reported control values, and 25% of control subjects have an amplitude ratio (palm/wrist) outside the reference range. Nevertheless, clinicians should consider stimulation of sensory nerves both proximal and distal to the site of compression (i.e., at the wrist and palm) to look for evidence of conduction block. This technique is less problematic than motor nerve stimulation in the palm, since it is unlikely that ulnar sensory responses will be volume-conducted to ring electrodes over the median innervated digits.
The electrodiagnostic examiner should also be aware of the Martin-Gruber anastomosis when performing median and ulnar motor conduction studies. An in-depth description of this can be found in the literature (17). Briefly, this represents the presence of an anomalous branch from the median nerve, or the anterior interosseus nerve, to the ulnar nerve in the proximal forearm. These crossing fibers typically innervate ulnar muscles, such as the first dorsal interosseus, but may also innervate hypothenar muscles or, rarely, thenar muscles. When stimulating the median nerve at the elbow, a larger response will be seen from the thenar recording electrodes than with wrist stimulation due to coactivation of nearby ulnar muscles via the crossing fibers. In CTS, the ulnar muscles supplied by the crossing fibers will be activated before the thenar muscles (which are slower due to demyelination at the wrist). Consequently, the elbow stimulation will produce an initial positive deflection and the measured conduction velocity in the forearm may be unusually fast. There is some evidence that recording from the first lumbrical (median innervated) and second palmar interosseus (ulnar innervated) muscles has an advantage over recording from the APB (18,19).
One should take special care in evaluating the patient with persistent symptoms after carpal tunnel release. Improvement in latencies usually occurs after successful release, with maximal improvement usually present within 6 months of surgery. However, latencies often do not return to the normal range (20) despite successful release.
Ulnar Neuropathy at the Elbow
CASE 2
A 46-year-old anesthesiologist has gradually noted mild but progressive weakness in his right hand and numbness in the ring and little finger. He comes to you at the prompting of a neurosurgical colleague who commented upon his wasting in the right first web space. The numbness is constant and involves both dorsal and palmar aspects of the little finger and palm, but does not extend proximal to the wrist. He denies any symptoms in the other upper limb or either lower limb. He denies neck pain except when working with selected surgeons in the operating room.
Physical examination reveals wasting in the hypothenar and first web space muscles. Strength is reduced (4/5) in finger abduction and adduction and in thumb adduction. Strength is otherwise normal, including wrist flexion and flexion of the distal interphalangeal joints. Sensation to pinprick is decreased over the little finger and ulnar aspect of the palm on both the dorsal and palmar surfaces. Muscle stretch reflexes at biceps, brachioradialis, and triceps are active and symmetrical.
The differential diagnosis includes ulnar neuropathy at the elbow, ulnar neuropathy at the wrist, and a lower brachial plexus lesion (lower trunk or medial cord) or C8 radiculopathy. The presentation is largely compatible with ulnar neuropathy at the elbow. The only findings that may at first glance appear inconsistent with this diagnosis are normal wrist flexion (in part supplied by the flexor carpi ulnaris) and normal flexor digitorum profundus strength. However, these muscles are often spared in ulnar neuropathy at the elbow. Ulnar neuropathy at the wrist is unlikely, given sensory involvement of the dorsal aspect of hand, supplied by the dorsal ulnar cutaneous nerve, which branches before the wrist. Normal strength in the thenar muscles suggests focal ulnar neuropathy rather than lower brachial plexopathy or C8 radiculopathy.
Clinical Presentation
Patients with ulnar neuropathy at the elbow typically report difficulty with sensation over the ulnar two digits of the hand. This involves both palmar and dorsal aspects of the little finger and usually the ulnar half of the ring finger. There is, however, substantial variability in the sensory supply to the hand. In up to 20% of patients there may be substantial variation where the ulnar nerve may supply the entire ring finger and the ulnar half of the middle finger, or just the small finger. Paresthesias are typically in the same distribution and usually do not extend above the wrist, although patients may report some elbow pain. Weakness is often less common than sensory complaints on initial report, but patients may have difficulty holding on to objects, especially difficulty with power grasp (such as using a hammer), since this requires using the hand in ulnar deviation.
On physical examination, sensory deficits are usually limited to the little finger and variably the ring finger and middle finger. Testing two-point discrimination is more sensitive than simply testing pinprick or light touch for detecting sensory deficits. In considering the differential diagnosis, it is important to accurately define the distribution of the sensory abnormalities. Patients with abnormal sensation limited to the palmar side of the hand are more likely to have an ulnar nerve lesion at the wrist, since the dorsal ulnar cutaneous nerve, supplying the back of the hand, branches from the ulnar nerve in the distal forearm (proximal to the wrist). If patients have sensory abnormalities extending into the medial forearm, the area supplied by the medial antebrachial cutaneous nerve, ulnar neuropathy is less likely. Since this sensory branch derives from the medial cord of the brachial plexus, patients with clear-cut sensory abnormalities over the medial forearm are more likely to have a lower brachial plexus lesion or cervical radiculopathy.
Atrophy may be observed in the first dorsal interosseus and hypothenar muscles, with sparing of the thenar musculature. The forearm usually appears normal. In more advanced cases, claw hand deformity may be present, with the medial two digits hyperextended at the metacarpophalangeal (MCP) joints and flexed at the interphalangeal joints. There is variability as to which fingers are involved in claw hand deformity, since there is often anatomic variation in which lumbricals are supplied by the ulnar and median nerves.
Weakness can be demonstrated in the dorsal or palmar interossei, as well as the adductor pollicis muscles. Froment’s sign, difficulty or inability to perform lateral pinch, is due to weakness of the adductor pollicis and flexor pollicis brevis, as well as the first dorsal interosseus. When patients are asked to perform a lateral pinch between the thumb and index fingers, they cannot do so, and as the strength of the pinch increases they compensate by using their long flexors (flexor pollicis longus and flexor digitorum profundus). Thus, instead of using the sides of the thumb and index finger to grasp an object such as a piece of paper, they use the tips of the fingers (known as Froment’s sign).
Special note should be made of examining the strength of the ulnar-innervated muscles in the presence of a radial neuropathy. Two errors are often made in this examination. First, many patients with isolated radial neuropathy are mistakenly thought to also have ulnar neuropathy because of weakness in finger abduction. This weakness is simply an artifact of the weak MCP joint extension produced by the radial neuropathy. In flexion, finger abduction is far weaker than it would be when the MCP joints are fully extended, due to a mechanical disadvantage. In the presence of a radial neuropathy, these ulnar-innervated muscles must be tested when the MCP joints are supported in full extension by placing the hand flat on a table or desk. Conversely, patients with a complete isolated radial neuropathy are often thought to have partial radial nerve sparing because they can extend the interphalangeal joints of the fingers. This, however, is a movement supplied by the ulnar nerve and does not indicate preservation of radial nerve function.
Many patients with ulnar neuropathy at the elbow have a positive Tinel sign over the elbow; however, many patients without ulnar neuropathy at the elbow will also test positive. While the Tinel sign is a sensitive finding on physical examination, it is not specific, and many patients without the disease also have a positive finding.
Nerve Conduction Studies
Motor nerve conduction studies are often the most useful technique for localizing the site of
ulnar neuropathy at the elbow and determining the pathophysiology of the lesion (Table 10-2). Recording from the abductor digiti minimi is the most commonly used method. Some authors, however, have found that recording from the first dorsal interosseus muscle, the most distal muscle supplied by the ulnar nerve, is more sensitive (21,22,23). A two-channel technique may be used to
record from both muscles simultaneously so that extra stimulations are not required. The recording site for the first dorsal interosseus is usually described as the active electrode over the bulk of the muscle, with the reference distally over the MCP joint of the index finger. Such a recording arrangement often produces an initial positive deflection, which is difficult to interpret. An initial negative deflection is more commonly seen when the reference is placed over the metacarpophalangeal joint of the thumb (see Fig. 9-14).
ulnar neuropathy at the elbow and determining the pathophysiology of the lesion (Table 10-2). Recording from the abductor digiti minimi is the most commonly used method. Some authors, however, have found that recording from the first dorsal interosseus muscle, the most distal muscle supplied by the ulnar nerve, is more sensitive (21,22,23). A two-channel technique may be used to
record from both muscles simultaneously so that extra stimulations are not required. The recording site for the first dorsal interosseus is usually described as the active electrode over the bulk of the muscle, with the reference distally over the MCP joint of the index finger. Such a recording arrangement often produces an initial positive deflection, which is difficult to interpret. An initial negative deflection is more commonly seen when the reference is placed over the metacarpophalangeal joint of the thumb (see Fig. 9-14).
Table 10-2 Nerve Conduction and Needle EMG Results for Case 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stimulation is usually performed at the wrist, below the elbow, above the elbow, and at the axilla (Fig. 10-3). While some electromyographers do not stimulate at the axilla routinely, the advantage of this technique is that it offers a conduction velocity across one more segment (the arm), which can be compared with the across-elbow conduction velocity. Study of the across-elbow segment requires much care in technique and interpretation. First, it is well known that the position of the elbow greatly influences the measured conduction velocity. When the elbow is extended, it is thought that the ulnar nerve may become redundant in the ulnar groove, and that surface measurements do not reflect the true distance of the underlying nerve. It is thought that flexing the elbow stretches the nerve to its full length, and measurement of the distance over the ulnar groove more closely reflects the distance along the nerve (see Fig. 9-14; Table 10-2) (24,25).
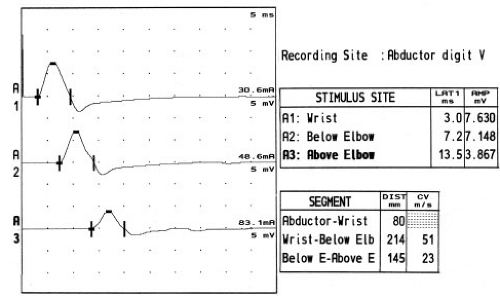 Figure 10-3 • Ulnar motor nerve conduction studies reported in Table 10-2. Stimulation of the ulnar nerve typically occurs at the wrist, below the elbow, and above the elbow. Conduction block (neurapraxia) at the elbow is shown by the decrease in CMAP amplitude with above-elbow stimulation. Some authors also recommend stimulation in the axilla (not shown). |
The distance between above- and below-elbow stimulation sites may also influence the accuracy of the conduction velocity measurement. Since surface measurements can be in error by many millimeters, use of short distances between stimulation sites means that there will be a relatively large percentage error in the distance and hence conduction velocity measurements. Many electromyographers recommend using at least a 10 cm across-elbow distance to reduce this measurement error (26), although recent data indicate that only 6 cm might be needed with the improved accuracy of today’s EMG instruments (27).
While Martin-Gruber anastomosis is usually discussed in the context of median nerve conduction
studies, it is probably far more important to recognize this anomaly when performing ulnar nerve conduction studies. When present, this anomaly will result in a much lower–amplitude response with below-elbow stimulation compared to that obtained with wrist stimulation. The inexperienced electromyographer may suspect a focal ulnar neuropathy in the proximal forearm, which could even be “confirmed” by inching studies as one inches along the ulnar nerve and across the anastomosis. However, in all such cases, the presence of a Martin-Gruber anastomosis can and should be ruled out very simply by stimulating the median nerve at the elbow and recording over the abductor digiti minimi and first dorsal interosseus muscles. Presence of any significant response with an initial negative takeoff indicates the presence of the anomaly.
studies, it is probably far more important to recognize this anomaly when performing ulnar nerve conduction studies. When present, this anomaly will result in a much lower–amplitude response with below-elbow stimulation compared to that obtained with wrist stimulation. The inexperienced electromyographer may suspect a focal ulnar neuropathy in the proximal forearm, which could even be “confirmed” by inching studies as one inches along the ulnar nerve and across the anastomosis. However, in all such cases, the presence of a Martin-Gruber anastomosis can and should be ruled out very simply by stimulating the median nerve at the elbow and recording over the abductor digiti minimi and first dorsal interosseus muscles. Presence of any significant response with an initial negative takeoff indicates the presence of the anomaly.
How much slowing in the across-elbow segment is sufficient to diagnose ulnar neuropathy? Some electromyographers compare the across-elbow velocity to the forearm velocity, allowing a difference of up to 11 to 15 m/s between the across-elbow and forearm segments before calling the finding abnormal (28). Other authors prefer to use the absolute conduction velocity rather than a comparison between segments (22,29). Comparison with the forearm segment assumes that the forearm segment remains normal in ulnar neuropathy at the elbow. However, with axon loss, the distal velocity often slows, making comparison less useful, which has led some to study the upper arm segment for comparison. A recent study has suggested that absolute velocities of less than 48 m/s are suggestive of ulnar neuropathy at the elbow and that this is superior to comparison with the forearm velocity (30).
Slowed conduction velocity is not the only finding that should be considered diagnostic of ulnar neuropathy at the elbow. Such patients may also have a drop in amplitude in the across-elbow segment or increased temporal dispersion. Some authors state that an amplitude reduction of more than 10% in the across-elbow 10 cm segment may be abnormal (28), but this is more convincing if accompanied by focal slowing or temporal dispersion.
Although it was just stated that a 10 cm minimum distance across the elbow is recommended for conduction velocity measurements, it is often found that study of very short segments yields a higher sensitivity for very focal lesions. With short-segment studies the injured segment with demyelination occupies a higher percentage of the distance studied, when compared to longer segments in which normal nerve dilutes the measurement. Inching studies (or perhaps more appropriately called centimetering studies) can be performed by stimulating the nerve at 2 cm increments across the elbow (31,32). Landmarks are best established by drawing a line between the medial epicondyle and the olecranon (33), and measuring 2 cm increments distal and proximal to this line. Stimulation must be carefully performed at just barely supramaximal, since overstimulation may cause nerve activation distal to the cathode and potentially distal to a lesion. Using this technique, a conduction delay of more than 0.7 ms across 2 cm segments is probably abnormal (31). More impressive are accompanying focal changes in amplitude or waveform morphology across a segment (Fig. 10-4).
Most of the abnormalities mentioned above require the presence of demyelination for localization. However, in many traumatic ulnar neuropathies in which there is only axon loss without demyelination, localization of ulnar neuropathy is far more difficult. In such cases, there will be diffuse mild slowing of conduction velocity without focal slowing, conduction block, or temporal dispersion; thus, there are no focal nerve conduction changes across the lesion. Needle EMG is only marginally helpful, since the two ulnar-innervated muscles in the forearm are often spared (see below) and there are no ulnar-innervated muscles in the arm. Therefore, despite one’s best technique, there are significant numbers of patients with primarily axonal lesions of the ulnar nerve in whom localization cannot be precisely determined.
Sensory nerve conduction studies are often of less localizing value than motor conduction studies. There are several technical problems that make this response more difficult to interpret. First, with stimulation of the ulnar nerve and antidromic recording over the little finger, there is often a large-amplitude hypothenar motor response volume conducted to the recording electrodes, which precludes accurate identification and measurement of the SNAP. Second, due to phase cancellation (34), the amplitudes of the sensory responses fall dramatically over distance, and reductions of 50% are not unusual or abnormal in the wrist-to-elbow
segment. Third, it is much harder to record sensory responses, particularly with proximal stimulation, when their amplitudes are reduced by significant ulnar neuropathy with temporal dispersion.
segment. Third, it is much harder to record sensory responses, particularly with proximal stimulation, when their amplitudes are reduced by significant ulnar neuropathy with temporal dispersion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree