Abstract
Management of lower urinary tract dysfunction (LUTD) in neurological diseases remains a priority because it leads to many complications such as incontinence, renal failure and decreased quality of life. A pharmacological approach remains the first-line treatment for patients with neurogenic LUTD, but electrical stimulation is a well-validated and recommended second-line treatment. However, clinicians must be aware of the indications, advantages and side effects of the therapy. This report provides an update on the 2 main electrical stimulation therapies for neurogenic LUTD – inducing direct bladder contraction with the Brindley procedure and modulating LUT physiology (sacral neuromodulation, tibial posterior nerve stimulation or pudendal nerve stimulation). We also describe the indications of these therapies for neurogenic LUTD, following international guidelines, as illustrated by their efficacy in patients with neurologic disorders. Electrical stimulation could be proposed for neurogenic LUTD as second-line treatment after failure of oral pharmacologic approaches. Nevertheless, further investigations are needed for a better understanding of the mechanisms of action of these techniques and to confirm their efficacy. Other electrical investigations, such as deep-brain stimulation and repetitive transcranial magnetic stimulation, or improved sacral anterior root stimulation, which could be associated with non-invasive and highly specific deafferentation of posterior roots, may open new fields in the management of neurogenic LUTD.
1
Introduction
Management of lower urinary tract dysfunction (LUTD) in neurological diseases remains a priority. Indeed neurological disorders such as multiple sclerosis and spinal cord injury (SCI) are responsible for neurogenic detrusor overactivity (NDO) associated with detrusor sphincter dyssynergia (DSD). DSD is characterized by involuntary detrusor contractions during the bladder-filling phase associated with lack of relaxation of the uretral-striated sphincter, which leads to incontinence, urinary retention and increased bladder pressure. The long-term result is upper urinary tract deterioration. Urinary incontinence can negatively affect quality of life (QoL). As well, patients with other neurological diseases such as Parkinson’s disease (PD) and stroke can experience urinary urgency, incontinence or retention. The prevalence of neurogenic LUTD depends on the type and duration of neurological diseases and could affect from half to all patients with neurological disorders depending on the terminology used to describe the LUT symptoms of interest .
The main goal of neurogenic LUTD management is regular, complete bladder emptying, avoiding high intra-detrusor pressure, and restoring continence so as to improve QoL and prevent complications. Current NDO management relies on pharmacotherapy, acting primarily at the level of the efferent motor micturition reflex branch, thereby allowing bladder filling at low pressure. First-line treatment combines oral antimuscarinics with or without intermittent bladder catheterization 5 to 6 times a day. However, the efficacy of antimuscarinics is limited, and atropinic side effects decrease compliance.
Electrical stimulation (ES) was developed as second- or third-line treatment for neurogenic LUTD and is now well validated and accepted. Indeed, all scientific societies have reported the interest of ES . However, despite many recent reviews related to these devices, several points need to be emphasized to provide the best information for the best prescription.
This report provides an update on ES management of neurogenic LUTD, focusing on the 2 main, although contrasting, therapies ( Table 1 ): stimulation to induce direct bladder contraction with the Brindley procedure and stimulation to modulate LUT physiology. We emphasize the several indications for the techniques in managing neurogenic LUTD, following international guidelines, with evidence of their efficacy in patients with neurologic disorders.
| Sacral anterior roots stimulation | Neuromodulation | ||
|---|---|---|---|
| Population | SCI (complete) | MS/PD/Stroke | |
| Indication | NDO + DSD | NDO | |
| Principle | Stimulation to induce detrusor contraction for voiding and posterior rhizotomy to achieve continence | Chronic stimulation to modulate micturition reflex | |
| Parameters of stimulation | Several pulses of stimulation of 300 milliseconds with a frequency of 30 Hertz, only when micturition is desired | Permanent (Interstim) or prolonged stimulation with waves of 200 μs and a frequency around 20 Hertz | |
| Reversibility | No | Yes | |
| Source of energy | External controller and powered by radio transmission | Intracorporeal batteries for interstim/extracorporeal batteries for PTN/DGN | |
| Site of stimulation | Intradurally | Extradurally | S3/pudendal/posterior tibial nerve/dorsal genital nerve |
2
Stimulation to induce direct detrusor contraction: sacral anterior root stimulation (SARS) and posterior root rhizotomy
2.1
History
Since the discovery in the 18th century of the connection between electricity and nerves, the collaboration between engineers and physicians has led to numerous efficient therapeutics. In the urological field, 2 major applications with two different principles were developed: stimulation to induce a direct effect and stimulation to modulate LUT physiology. For more than a century, many sites have been stimulated, from the conus medullaris to the pelvic floor and the detrusor. Researchers have applied the technology in various pathologies, which has progressively led to clear clinical indications. The major applications are the result of tremendous worldwide research efforts since the 1950s.
Urinary retention due to detrusor areflexia was a real challenge before the development of intermittent catheterization. In 1955, MacGuire used various electrodes to stimulate the detrusor in dogs . Ten years later, Bradley et al. developed an implantable stimulator with 2 circular electrodes placed laterally to the detrusor. However, only 2 of the 7 paraplegic patients could void properly. Another approach was to use a metal electrode inserted on an indwelling catheter.
Long-term chronic ES allows for achieving detrusor contraction. This technique was developed by a Danish surgeon to treat chronic urinary retention after pelvic surgery. From these results, Madersbasher et al. used the same type of chronic intravesical stimulation in patients with myelomeningocele . Direct ES of the pelvic floor was also used to treat stress incontinence by various teams, for example, Caldwell in 1963 . Other sites were tested with mixed results and major technical problems; one site tested in 1971 was the spinal cord to stimulate the parasympathetic center. The major advance was by Brindley, in 1977 , who combined SARS and posterior radicotomy to treat urinary incontinence and micturition disorders in patients with SCI.
2.2
Indication
SARS with posterior root rhizotomy can be proposed as a validated option for managing NDO and dyssynergia in patients with SCI because of research with long-term follow-up (grade B evidence) . Patients need to be properly assessed before implantation because of the irreversibility of the approach. The technique can be performed only in patients with SCI with preserved sacral reflex and normal detrusor compliance. Because all or a part of the posterior sacral nerves are destroyed, the technique cannot be performed in patients with conserved lower limb motility (grade B evidence) . Moreover, this deafferentation induces sensory loss in the sacral dermatomes and patients could lose reflexogenic erection. The principal indication is for achieving urine continence and bladder voiding without catheterization, even if this stimulation can also help with defecation and erection.
2.3
Description of the technique
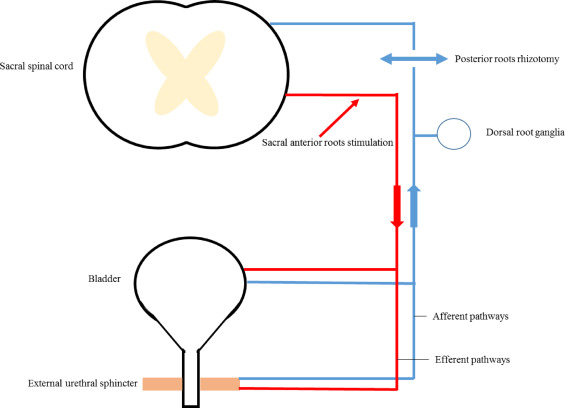
Brindley described SARS in the 1970s, with the first human implantations in 1972 . The patient is under general anesthesia to avoid drugs interfering with bladder contraction, which needs to be recorded during surgery. Three approaches have been described: intradural, extradural and sacral . The technique is separated into 3 parts. First, sacral roots are stimulated to identify the motor (anterior) and sensory (posterior) roots and to determine those involved in micturition by detrusor pressure monitoring. Then 3 intracorporeal parts, including 4 slots with 3 platinum foil electrodes, 3 cables and a receiver block, are implanted. The electrodes can be placed extradurally or intradurally on the sacral roots from S2 to S4. Finally, the implantation is accompanied by a larger posterior rhizotomy, to S4 . This rhizotomy, with its functional consequences, allows for complete detrusor areflexia and normal bladder compliance ( Fig. 1 ).
2.4
Mechanism of action
The purpose of the procedure is to allow for continence and micturition. The micturition is obtained by several pulses of stimulation of 300 ms at 30 Hz . These intermittent stimulations induce a prolonged contraction of the detrusor smooth muscle while striated fibres of the external urethral sphincter undergo periods of relaxation during the “off” phase of intermittent stimulation. This difference in relaxation and contraction time between striated and smooth fibres associated with posterior rhizotomy prevents active dyssynergia. Patients often need several courses of stimulation and subsequent flow to empty the bladder . Dorsal root rhizotomy, extended to S4, interrupts afferent synaptic transmission from the bladder, which is responsible for the exacerbated micturition reflex with incontinence after SCI. Because the efferent sacral somatic pathways are preserved, the surgery induces no stress incontinence.
2.5
Results and limitations in neurogenic disorders
SARS associated with posterior rhizotomy is an effective technique after implantation. Indeed, the procedure can decrease urinary-tract infection prevalence (68%), improve continence (54%) and thus improve social life (54%) . Moreover, a cystometric study found significant amelioration of elevated detrusor pressure, low detrusor compliance and limited capacity in patients with SCI . An important advantage of the technique is restoration of micturition without catheterization, with preservation of the upper urinary tract . Despite the efficacy, the technique is not commonly used because of the irreversibility and consequences of the posterior rhizotomy as well as loss of reflex erection or vaginal lubrification but also sensory loss in sacral dermatomes. Moreover, accidental lesion of sacral motor roots could lead to permanent incontinence and retention. Thus, only 2000 procedures have been performed since the 1970s. A new approach of non-invasive and highly specific deafferentation limited to bladder afferences would provide continence and micturition without side effects, for a real breakthrough in management of NDO.
2
Stimulation to induce direct detrusor contraction: sacral anterior root stimulation (SARS) and posterior root rhizotomy
2.1
History
Since the discovery in the 18th century of the connection between electricity and nerves, the collaboration between engineers and physicians has led to numerous efficient therapeutics. In the urological field, 2 major applications with two different principles were developed: stimulation to induce a direct effect and stimulation to modulate LUT physiology. For more than a century, many sites have been stimulated, from the conus medullaris to the pelvic floor and the detrusor. Researchers have applied the technology in various pathologies, which has progressively led to clear clinical indications. The major applications are the result of tremendous worldwide research efforts since the 1950s.
Urinary retention due to detrusor areflexia was a real challenge before the development of intermittent catheterization. In 1955, MacGuire used various electrodes to stimulate the detrusor in dogs . Ten years later, Bradley et al. developed an implantable stimulator with 2 circular electrodes placed laterally to the detrusor. However, only 2 of the 7 paraplegic patients could void properly. Another approach was to use a metal electrode inserted on an indwelling catheter.
Long-term chronic ES allows for achieving detrusor contraction. This technique was developed by a Danish surgeon to treat chronic urinary retention after pelvic surgery. From these results, Madersbasher et al. used the same type of chronic intravesical stimulation in patients with myelomeningocele . Direct ES of the pelvic floor was also used to treat stress incontinence by various teams, for example, Caldwell in 1963 . Other sites were tested with mixed results and major technical problems; one site tested in 1971 was the spinal cord to stimulate the parasympathetic center. The major advance was by Brindley, in 1977 , who combined SARS and posterior radicotomy to treat urinary incontinence and micturition disorders in patients with SCI.
2.2
Indication
SARS with posterior root rhizotomy can be proposed as a validated option for managing NDO and dyssynergia in patients with SCI because of research with long-term follow-up (grade B evidence) . Patients need to be properly assessed before implantation because of the irreversibility of the approach. The technique can be performed only in patients with SCI with preserved sacral reflex and normal detrusor compliance. Because all or a part of the posterior sacral nerves are destroyed, the technique cannot be performed in patients with conserved lower limb motility (grade B evidence) . Moreover, this deafferentation induces sensory loss in the sacral dermatomes and patients could lose reflexogenic erection. The principal indication is for achieving urine continence and bladder voiding without catheterization, even if this stimulation can also help with defecation and erection.
2.3
Description of the technique
Brindley described SARS in the 1970s, with the first human implantations in 1972 . The patient is under general anesthesia to avoid drugs interfering with bladder contraction, which needs to be recorded during surgery. Three approaches have been described: intradural, extradural and sacral . The technique is separated into 3 parts. First, sacral roots are stimulated to identify the motor (anterior) and sensory (posterior) roots and to determine those involved in micturition by detrusor pressure monitoring. Then 3 intracorporeal parts, including 4 slots with 3 platinum foil electrodes, 3 cables and a receiver block, are implanted. The electrodes can be placed extradurally or intradurally on the sacral roots from S2 to S4. Finally, the implantation is accompanied by a larger posterior rhizotomy, to S4 . This rhizotomy, with its functional consequences, allows for complete detrusor areflexia and normal bladder compliance ( Fig. 1 ).










