Abstract
Objective
In hemiplegic children the appearance of equinovarus is correlated with premature electromyography (EMG) activity of the gastrocnemius medialis (GM) prior to initial contact. The goal was to analyze the onset of EMG activation in the GM and, more particularly, the peroneus longus (PL) in cases of equinovarus: is PL activity likewise premature?
Material and methods
As 15 hemiplegic children (age 5 years ± 1.5) with equinovarus walked, their PL and GM EMG activity was being recorded. The latter was normalized in terms of gait cycle percentage (0–100%) and detected through semi-automatic selection with activation threshold set at 20 μV. A paired t -test compared activation onset of the PL versus the GM muscles.
Results
As regards the healthy limb, activity onset of the GM (+14.55%) and the PL (+19.2%) muscles occurred only during the ST. In cases of equinovarus, activation of the GM (−5.2%) and the PL (−6.1%) occurred during the SW and was premature. For each muscle, comparison between the healthy and the hemiplegic side was highly significant ( P < 0.001).
Conclusion
Premature PL and GM EMG activity preceding initial contact corresponds not to a disorder secondary to imbalance but rather, more probably, to motor command dysfunction. While the PL consequently contributes to equinus deformity, its possible role in varus genesis is less evident. EMG study needs to be completed by comparing PL and tibialis posterior strength while taking foot bone morphology into full account.
Résumé
Objectif
L’équin varus chez l’enfant hémiplégique est corrélé à une activation EMG prématurée du gastrocnemius medialis (GM). Le but était d’analyser le début d’activation EMG du GM et du peroneus longus (PL) dans l’équin varus : le PL est-il prématuré ?
Matériel et méthode
Quinze hémiplégiques (âge 5 ± 1,5 ans) marchant en équin varus ont eu un EMG PL et GM. L’activité EMG (0–100 %) était normalisée en pourcentage du cycle de marche et déterminée par une méthode semi-automatique avec un seuil d’activation de 20 μV. Un test de Student comparait le début d’activation du PL versus GM.
Résultats
Côté sain, le début de l’activation du GM (+14,55 %) et du PL (+19,2 %) se faisait uniquement en phase d’appui (NS). Côté hémiplégique, GM −5,2 % et PL −6,1 % (NS) étaient prématurés en phase d’oscillation. Pour chaque muscle, la comparaison sain versus hémiplégique était très significative ( p < 0,001).
Conclusions
Cette activité prématurée EMG PL et GM précédant le contact initial ne correspond pas à un trouble secondaire à un déséquilibre mais probablement à un trouble de la commande. Le PL participe donc à l’équin mais il est difficile d’affirmer son rôle dans la genèse du varus. Il faut compléter l’étude EMG avec le tibialis posterior, comparer leur force et prendre en compte l’aspect constitutionnel (osseux).
1
English version
1.1
Introduction
Gage has described 5 factors enabling effective and functional gait; one of them is correct pre-positioning of the foot at the end of the swing phase (SW), which prepares initial contact (IC) by the heel. When this condition is not met, there exists an equinus .
In the hemiplegic child with cerebral palsy (CP), equinus deformities are likely to develop as the diseased foot continues to grow. But before the bone deformities are set into place, abnormal electromyography (EMG) activity sequences for the gastrocnemius medialis (GM), as well as other muscles, are likely to trigger equinus. This will occur at a very early stage, while the infant is learning to walk ; during the SW, EMG activation of the GM is premature. Early EMG in the cerebral palsy child is particularly instructive. In cases of equinovarus, premature GM and tibialis posterior (TP) activation has been observed repeatedly . More precisely, premature EMG activity has been shown to occur at the end of the SW; one of Gage’s conditions for functional gait has not been met ; IC will involve not the heel but the fifth metatarsal head, which means that equinovarus has come into being.
As concerns equinovarus in a CP child, Perry took note of EMG activation during the SW of the PL, and it was deemed paradoxical due to the action of the equinovalgus deformity of the PL . However, Perry had neither studied nor proposed a pathophysiological explanation for his observation.
While examining hemiplegic CP children in our laboratory, we made the same EMG-based clinical observation. That is why the objective of this study was to evaluate and quantify the EMG activation sequence of the PL and GM muscles in hemiplegic children presenting with equinovarus. Indeed, to our knowledge the EMG activation sequence common to PL and GM in hemiplegic CP children has yet to be described in any published study. We wished to determine whether or not the synergic action of PL and GM in cases of equinovarus should be put forward as a hypothesis.
1.2
Materials and method
We extracted from our database a series of CP children who had been given a dynamic EMG analysis.
1.2.1
Clinical data
Our retrospective study was carried out from 2008 until 2011. All of the children were monitored by our pediatric neurology unit. Inclusion criteria were: CP diagnosis by a child neurologist (B.C.), child under 6 years of age, right or left hemiplegia, good functional level (Gross Motor Function Classification System 1 [GMFCS 1], validated only for children aged from 6 to 12 years). Clinical examination and video images allowed for definition of the positions of the ankle and the forefoot during IC. In clinical examination of the plantar side of the feet, reproducibility of the equinovarus was revealed by hyperkeratosis face to the 5th metatarsal head (M5), which objectified observation of preferred M5 placement. According to Wren et al. , equinus is defined by ankle plantar flexion lower than 0°. The key equinovarus criterion was IC with the M5 ( Fig. 1 ). A posterior view shows the position of the forefoot as initial M5 contact is taking place ( Fig. 1 ).

Children presenting with equinovarus foot deformity confirmed by two therapists (a physical and rehabilitation medicine specialist and a physiotherapist) were included in the study. Goniometric evaluation aimed at measuring dorsiflexion amplitudes of the ankle between tibia and hindfoot was performed by two practitioners (C.B. and G.A.) with the child placed in a supine position, his or her knees first bent and then stretched; care was taken to avoid pronation or supination of the forefoot. Criteria of exclusion consisted in an equinus deformity involving retraction of the triceps sural (calf muscle) and a difference in length of the lower limbs to the detriment of the hemiplegic side greater than 1 cm. Fifteen hemiplegic children were included: 9 with deformities on the left and 6 on the right; 7 girls and 8 boys. Average age was 5 ± 1.5 years. The mean number of gait cycles analyzed was 15 ± 10. The children walked barefooted in accordance with their spontaneous walking velocity. Some authors have demonstrated that muscle activation depends on speed. That is why, in order to optimally compare the hemiplegic side with the healthy side, we opted in favor of the most easily reproducible conditions: shoeless and with the speed specific to each child (rather than an imposed pace).
1.2.2
Data acquisition
The children were given an EMG test of the healthy side and the hemiplegic side. The EMG data were recorded through use of a Wifi system (Zero Wire Aurion, Italy). The EMG signals were picked up at an acquisition frequency of 2000 Hz, amplified 1000×, filtered (10 Hz high-pass filter, 500 Hz low-pass filter) and rectified.
The skin had been preliminarily degreased, stripped and cleaned. Two electrodes (Ag-AgCl, diameter 10 mm) were positioned parallel to the PL fibers at ¼ of a line running from the fibular head to the edge of the lateral malleolus. The SENIAM project (Surface EMG for Non-Invasive Assessment of Muscles) recommends a distance of 10 mm between the electrode centers. Standard SENIAM protocol was scrupulously respected in view of minimizing interference or cross-talk. More specifically, validation of electrode positioning was aimed at minimizing cross-talk between not only GM and PL but also PL and tibialis anterior (TA), and it was carried out first by plantar flexion movements with stretched knees (GM testing) and eversion (PL), and then by dorsal inversion-flexion (TA). The objective of the validation procedure was to observe asynchronicity in the bursts of EMG activity during phases of voluntary movement. EMG activity onset was identified through use of a semi-automatic method; the EMG signal for a given muscle was defined as active when the rectified raw signal exceeded 20 microvolts . Indeed, 20 microvolts is the commonly recognized threshold usually applied when studying motor unit potential and attempting to exclude any artifact.
1.2.3
Data analysis
The EMG activation sequences were analyzed in terms of timing with regard to the gait cycle. Muscle activity was normalized as gait cycle percentage. Onset of the activation sequence for the PL and GM muscles was expressed according to the latter (0–100%). Perry has defined premature GM activity as beginning during the SW, which means prior to IC; at this stage the GM muscle presents no EMG activity in healthy children. Using the same method, Perry defined onset of premature PL activity during the SW preceding IC; the PL shows no activity in healthy children at this stage.
Premature EMG activation of the GM and PL muscles was consequently normalized and expressed as gait cycle percentage. By convention, EMG activation occurring prior to IC (0%) was noted in negative terms; all premature activity was considered as negative. For example, if premature activity commenced at 10% before IC, it was identified as −10%. The onset of PL and GM activation could consequently be studied and compared. A paired t -test was used. Activation onset was compared between PL and GM muscles and between the hemiplegic and the healthy sides. Activation onset for PL and GM on the healthy side was compared to the data reported by Perry , which were considered as references.
Normal distribution of the parametric variables was verified by the Kolmogorov-Smirnov test and by equality of variance tests in order to ensure that the conditions required for application of the comparison of means (Student’s) test had been fulfilled. Statistical significance was set at P < 0.05.
1.3
Results
1.3.1
The clinic
The mean for ankle dorsiflexion (bended knees) was 25 ± 5° on the hemiplegic side and 28 ± 9° on the healthy side ( P < 0.05). The mean for ankle dorsiflexion (stretched knees) was 15 ± 10° on the hemiplegic side versus 20 ± 8°on the healthy side ( P < 0.05) ( Table 1 ).
| Healthy side ( n = 15) Mean ± S.D. | Hemiplegic side ( n = 15) Mean ± S.D. | |
|---|---|---|
| Dorsiflexion ankle | ||
| Bended knee | 20 ± 5° | 15 ± 5° |
| Stretched knee | 18 ± 4° | 14 ± 7° |
| Initial contact | Heel | M5 |
As regards the length of each lower limb, mean intra-observer variability was 3 mm; with an extra-observer, it rose to 5 mm.
ST duration was 62% on the healthy side and 55% on the hemiplegic side.
1.3.2
Electromyography
On the healthy side, mean PL activation onset occurred at 19.2% of the gait cycle, while on the hemiplegic side, the analogous mean was −6.1% ( P < 0.001) ( Table 2 ; Fig. 2 ). The PL muscle was prematurely activated during the SW.
| Activation onset | Healthy side | Hemiplegic side | t -test | |
|---|---|---|---|---|
| Activation sequence | Normal | Prematurity | ||
| GM (mean/S.D.) | +14.55% / 11 | −5.2% / 12.4 | P < 0.001 | S*** |
| PL (mean/S.D.) | +19.2% / 11.8 | −6.1% / 15.1 | P < 0.001 | S*** |
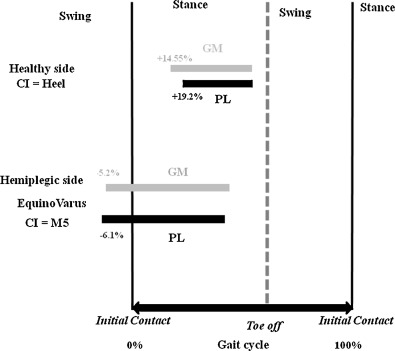
On the healthy side, mean GM activation onset occurred at 14.55% of the gait cycle, while on the hemiplegic side, the analogous mean was −5.2% ( P < 0.001) ( Table 2 ) ( Fig. 2 ). The GM muscle was prematurely activated during the SW.
Both the PL and the GM muscles showed premature activity with regard to IC, but presented no significant difference as concerns activation onset ( Table 3 ; Fig. 2 ).
| Activation onset | Hemiplegic side | t -test | |
|---|---|---|---|
| Activation sequence | Prematurity | ||
| GM (mean/S.D.) | −5.2% / 12.4 | ||
| PL (mean/S.D.) | −6.1% / 15.1 | P = 0.8 | NS |
1.4
Discussion
In a population of hemiplegic children, the presence of an equinus can be attributed to premature EMG activity of the GM muscle especially. Indeed, EMG activations have been documented in equinovarus with regard to the varus muscles. Abnormal premature PL activation has already been described in hemiplegic children with equinovalgus deformity . In addition, Perry observed premature EMG activation of the PL muscle during the SW in cases of equinovarus diagnosed amongst a population of CP children. Our results quantify the prematurity and the observations made by Perry.
As in any pediatric study, especially with very young patients, variability of gait cycles may constitute a bias, as could the small population size ( n < 30) and the absence of a control group.
Moreover, interpretation of the EMG dynamic in pediatrics necessitates a number of references to the literature. For some authors, muscle activation sequences are considered as mature from the age of 2 years . While Sutherland et al. described highly exceptional premature activation sequences in children from 2 to 7 years of age, the population studied by his team was very small. Moreover, exceptional degrees of prematurity were not confirmed by Shiavi et al. , who did not determine a difference between the ages of 4 and 11 years. More recently, Granata et al. demonstrated that EMG activation sequences pertaining to the lower limbs came into play well before the age of 7 years.
In our study, stance phase (ST) duration on the healthy side (toe off 62%) was in accordance with the data in the literature . On the same token, diminution of the ST on the hemiplegic side (toe off 55%) signifies the avoidance strategy or transfer of the center of gravity to the healthy side that has been described in the CP child . Gait cycle variability in children was evaluated through selection of the most repeatable and reproducible cycles. During IC, equinovarus was defined as contact with the 5th metatarsal head ( Fig. 1 ) without the gradual knee bending that could have provoked its occurrence, which is seldom observed in hemiplegic patients. From a clinical standpoint and in the video images, the lower limbs presented no torsional abnormality, nor did any retraction of the triceps sural muscle occur. Nevertheless, ankle dorsiflexion was significantly diminished on the hemiplegic side, which meant that early modifications of the capusloligamentous, musculoaponeurotic and tendinous structures had indeed taken place. Since the latter are a source of passive resistance to dorsiflexion, they contribute to development and fixation of the equinus.
According to the data in the literature, normal GM and PL activation sequences take place only during the ST on the healthy side . The two muscles show no activity during the SW, which means that previous findings have been corroborated in our study ( Table 2 ). As regards the hemiplegic side, activation onset is premature ( Table 2 ), but there is no significant difference in prematurity between the GM and PL muscles. In the literature, the main cause of equinovarus is overactivity involving primarily the triceps sural, and also the TP.
The premature EMG activity of the PL during SW, which means in an open kinetic chain, does not correspond to an activation disorder secondary to compensation or disequilibrium, which is observed during ST in a closed kinetic chain as a means of stabilizing the foot and the ankle. As a result, premature PL and GM activity in the SW would more likely be due to a problem pertaining to primary EMG activation, i.e. to misdirected commands in CP cases. Premature synergic PL and GM activity could consist in spastic co-contractions of the two muscles. It has been shown that such co-contraction is one of the forms of muscle overactivity interfering with walking in a spastic paresis subject. Gracies et al. attributed spastic co-contraction to a misdirected descending command from dorsiflexor to plantar flexor, as is seen in CP; exaggerated contraction of the plantar flexors of the ankles (PL and GM) is likely to be triggered by the ankle dorsiflexion effort observed during SW.
To sum up, in synergy with the GM, the PL muscle strongly contributes to occurrence of equinus during SW, and the deleterious effects of this action may tend to be underestimated . That said, and even though Thevenon et al. mention this type of causation with in equinovarus in a hemiplegic adult after an ischemic stroke, it would appear problematic to underline the role of the PL in the genesis of the varus. While this muscle exerts an locking action on the midfoot in the middle of the ST accompanying the 1st rocker, it does not act upon the tibiotarsal joint . On the other hand, the PL provides support for the longitudinal and transversal arches of the foot. At the end of the ST during the 3rd rocker for the push-off, the PL again exerts locking action, this time on the 1st and 2nd rays i.e. the medial fore foot; the forefoot is stabilized as the heel is loosened . So it is that the PL brings about forefoot pronation as a means of compensation for the action of the varus muscles (essentially the GM and the TP) . In equinus, however, the PL relinquishes its function as stabilizer and support provider of the 1st ray .
To conclude, further pathophysiological understanding entails conjoined exploration of PL and TP muscles in terms of EMG activation sequences, particularly with reference to the co-contraction indexes involving the two muscles, the TA recruitment index and a strength index, as well. The other key element to be taken into account is the constitutional or osseous aspect, which should be assessed both clinically (forefoot, mid- and hindfoot) and radiographically, so that foot morphology development can be monitored during growth. Westberry et al. have described the pediatric radiographic norms in the hemiplegic child through which it is possible to quantify morphology of the forefoot (abduction/adduction, planus/cavus), midfoot (abduction/adduction, pronation/supination) and hindfoot (calcaneus/equinus, valgus/varus).
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
2
Version française
2.1
Introduction
Gage a décrit 5 facteurs pour une marche fonctionnelle et efficace dont le pré-positionnement correct du pied en fin de phase d’oscillation (PO) afin de préparer un contact initial (CI) par le talon. Si ce pré-requis n’est pas respecté, il existe un équin .
Chez l’enfant hémiplégique par paralysie cérébrale (PC), les déformations dues à l’équin ont un potentiel d’évolutivité étant donné la croissance du pied à venir. Avant que les déformations osseuses ne soient fixées, ce sont les troubles de la séquence d’activation électromyographique (EMG) du gastrocnemius medialis (GM), entre autres, qui vont entraîner l’équin, et ce de façon très précoce lors de l’acquisition de la marche : la séquence d’activation EMG du GM est prématurée en PO. L’EMG précoce chez le jeune enfant atteint de PC est très informatif. Dans l’équin varus, une activation prématurée du GM et du tibialis posterior (TP), essentiellement, est constatée . En effet cette activation EMG prématurée se fait en fin de PO. Un des pré-requis de Gage n’est pas respecté : le CI ne se fera pas par le talon mais sur la tête du 5 e métatarsien, un équin varus est constitué.
Dans l’équin varus chez l’enfant PC, Perry avait constaté la présence paradoxale d’une activation EMG en PO du PL : action paradoxale étant donné l’action d’équin valgus du PL . Cependant Perry n’avait pas étudié ni donné d’explication physiopathologique à ce constat.
Lors des évaluations des enfants hémiplégiques par PC au sein de notre laboratoire, nous avons fait cette même constatation clinique et EMG. C’est pourquoi le but de ce travail était d’évaluer et de quantifier la séquence d’activation EMG du PL et du GM chez les enfants hémiplégiques présentant un équin varus. Cette étude de séquence d’activation commune PL et GM n’a pas été décrite chez l’enfant hémiplégique par PC. L’hypothèse était de mettre en évidence ou non l’action synergique du PL avec le GM dans l’équin varus.
2.2
Matériels et méthode
Nous avons extrait de notre banque de données une série d’enfants PC qui avaient bénéficié d’une analyse EMG dynamique.
2.2.1
Données cliniques
Il s’agissait d’une étude rétrospective entre 2008 et 2011. Tous les enfants étaient suivis dans le service de neurologie pédiatrique. Les critères d’inclusion étaient : le diagnostic de PC avait été réalisé par un neuropédiatre (B.C.), un âge inférieur à 6 ans, une hémiplégie droite ou gauche, un bon niveau fonctionnel (Gross Motor Function Classification System 1 [GMFCS 1], validé uniquement pour les enfants âgés de 6 à 12 ans). L’examen clinique et la vidéo permettaient de définir la position de la cheville et de l’avant-pied lors du CI. Lors de l’examen clinique de la face plantaire des pieds, la reproductibilité de l’équin varus était révélée par l’hyperkératose en regard de la tête de M5 objectivant l’appui préférentiel à ce niveau sur la tête M5. Selon Wren et al. , l’équin est défini comme une flexion plantaire de la cheville en dessous de 0°. Le critère de l’équin varus était un CI qui se faisait avec la tête du 5 e métatarsien (M5) ( Fig. 1 ). Une vue postérieure lors du CI révèle la position de l’avant-pied lors du contact initial par M5 ( Fig. 1 ).










