Abstract
Objective
There are no handbook or recommendations for the use of pharmacological agents to treat neurobehavioral disorders after traumatic brain injury (TBI). This work proposes a systematic review of the literature and a user guide on neuroleptics, antidepressants, beta-blockers, mood stabilizers and other medications for irritability, aggressiveness, agitation, impulsivity, depression, apathy…
Method
Steering, working and reading groups (62 people) were formed under the control of the French High Authority for Health (HAS) in collaboration with the SOFMER scientific society (French Society of Physical and Rehabilitation Medicine). Articles were searched by HAS officers in the Medline database from 1990 to 2012, crossing TBI and pharmacological agents. The HAS method to select, read and analyze papers is close to the PRISMA statements.
Results
Out of 772 references, 89 were analyzed, covering a total of 1306 people with TBI. There is insufficient evidence to standardize drug treatments for these disorders. There are however some elements to establish consensus recommendations for good clinical practice. Propranolol can improve aggression (B grade). Carbamazepine and valproate seem effective on agitation and aggression and are recommended as first line treatment (Expert Consensus [EC]). There is no evidence of efficacy for neuroleptics. Their prescription is based on emergency situation for a crisis (loxapine) but not for long-term use (EC). Antidepressants are recommended to treat depression (EC) with a higher standard of proof for Selective Serotonin Reuptake Inhibitors (SSRI, grade B). Other products are described.
Conclusion
The choice of treatment depends on the level of evidence, target symptoms, custom objectives, clinical experience and caution strategies.
1
Introduction
Behavioral disorders after traumatic brain injury (TBI) represent the main impairment for patients after their accident . The care management of these behavioral disorders is highly relevant for families and society. Behaviors, such as agitation, opposition, disinhibition, irritability, impulsiveness, bulimia, hypersexuality, Kluver and Bucy Syndrome, hostility, aggressiveness, verbal and physical violence, anxiety and depression (see Stephan et al. in this issue) require the consensus from experts who understand the specific characteristics of people with TBI. The pharmacological approach is highly specialized and is based on a comprehensive clinical experience. The most recent data from international literature suggest using beta-blockers, neuroleptics, antiepileptics, antidepressants, benzodiazepines, amantadine and other drugs.
The SOFMER French Society of Physical Medicine and Rehabilitation under the auspices of the French High Authority for Health (HAS) decided to elaborate recommendations of good practice (RGP), in response to the announcement in 2010 of a specific government action plan for patients with TBI. Through a systematic review of the literature, the objective of this work was to organize care pathways, provide a practical care management guide and improve the effectiveness of therapeutic modalities. These recommendations concern adult patients with traumatic brain injury presenting with behavioral disorders in the acute and chronic post-traumatic stages. These patients are still hospitalized or living at home or in an institution. The professionals concerned are physicians, healthcare personnel from the units caring for these patients, personnel of medico-social institutes or specialized care networks.
The population of patients with TBI is more sensitive to pharmacological treatments, it is a particular population and it deserves specific studies that are difficult to implement in randomized, double-blind vs. placebo protocols. Multicenter studies are often necessary to obtain a sample of patient large enough and homogeneous to obtain sufficient statistical power (e.g. age, time since injury, identical measure scales and identical concept definitions). Almost all systematic review studies, controlled or not-controlled studies and original studies come to the conclusion that further studies with a better methodology are needed. The relevance of this work is a dual one. On the one hand, proposing a systematic review of the literature to provide therapeutic solutions according to the available level of evidence and on the other hand bring consensus expert opinions when studies are insufficient to draw a conclusion.
2
Methodology
According to HAS criteria, the methodology involved a total of 62 people divided into 3 working groups, and 4 stages:
- •
elaboration of a framework letter with questions developed by the Steering committee (6 people: 3 professors of PM&R, 1 lawyer, 1 director of a medical structure);
- •
selection, analysis of the scientific literature and elaboration of a scientific rationale by the project managers (8 people: 1 librarian, 2 HAS physicians, 1 PM&R professor, 4 PM&R physicians);
- •
the elaboration of recommendations, based on the scientific rationale, by a working group (23 people: 5 project leaders PM&R physicians, 3 psychologists, 2 people representing families, 4 PM&R physicians, including 1 professor, 4 psychiatrists, 1 director of a medical structure, 1 professor of physical education, 1 MDPH (Departmental Home for Disabled Persons) physician, 1 social worker, 1 lawyer);
- •
the critic analysis of all proposals by a reading group (30 people: 7 psychologists, including 3 professors, 10 PM&R specialists, including a professor, a magistrate, a lawyer, a physiotherapist, a social worker, 2 healthcare managers, 2 people representing the families, one person representing the insurance companies, one director of a medical structure, a psychiatrist, a physician working in the prison system).
The HAS methodology is explained in details in this special issue, in the editorial (see Mathé and Luauté). This editorial reports 6 questions, our work focuses on drug therapeutics.
The literature research was performed by the HAS literature research team using as the main database Medline over the 1990–2012 period. Some additional articles related to the final selection but anterior to 1990 were also analyzed. Literature search strategies are detailed in Box 1 . A complimentary search was performed covering the period up to June 2015 without using the HAS research team. Each article selected was analyzed according to the literature review methodology using reading grids in order to attribute to each article a scientific level of evidence . According to the level of evidence of the studies on which they recommendations are based, they have a variable grade, scored from A to C, see Table 1 .
(“Brain Injuries” (Majr: NoExp) OR “Craniocerebral Trauma” (Majr: NoExp) AND “Drug Therapy” (Mesh) Or “Central Nervous System Stimulants” (Mesh) OR “Methylphenidate” (Mesh) OR “Dopamine Agents” (Mesh) OR “Dopamine” (Mesh) OR “Amantadine” (Mesh) OR “Dopamine Agonists” (Mesh) OR “Bromocriptine” (Mesh) Or “Levodopa” (Mesh) OR “Antidepressive Agents” (Mesh) OR “Sertraline” (Mesh) OR “Fluoxetine” (Mesh) OR “Paroxetine” (Mesh) OR “Citalopram” (Mesh) OR “tianeptine” (Supplementary Concept) OR “Trazodone” (Mesh) OR “Amitriptyline” (Mesh) OR “Clomipramine” (Mesh) OR “Trimipramine” (Mesh) OR “Mianserin” (Mesh) OR “mirtazapine” (Supplementary Concept) OR “milnacipran” (Supplementary Concept) OR “duloxetine” (Supplementary Concept) OR “Iproniazid” (Mesh) OR “venlafaxine” (Supplementary Concept) OR “Cholinesterase Inhibitors” (Mesh) OR “Physostigmine” (Mesh) OR “donepezil” (Supplementary Concept) OR “rivastigmine” (Supplementary Concept) OR “Adrenergic beta-Antagonists” (Mesh) OR “Propranolol” (Mesh) OR “Haloperidol” (Mesh) OR “Methotrimeprazine” (Mesh) OR “Clozapine” (Mesh) OR “quetiapine” (Supplementary Concept) OR “ziprasidone” (Supplementary Concept) OR “Anticonvulsants” (Mesh) OR “Valproic Acid” (Mesh) OR “Carbamazepine” (Mesh) OR “lamotrigine” (Supplementary Concept) OR “Lithium” (Mesh) OR “zolpidem” (Supplementary Concept) OR “modafinil” (Supplementary Concept) OR “Brain Injuries/drug therapy” (Majr) OR “Craniocerebral Trauma/drug therapy” (Majr) AND “Meta-Analysis as Topic” (Mesh) OR “Meta-Analysis” (Publication Type) OR “Review Literature as Topic” (Mesh) OR Meta-Analysis OR Review Literature Or Quantitative Review OR “Random Allocation” (Mesh) OR “Randomized Controlled Trials as Topic” (Mesh) OR “Randomized Controlled Trial” (Publication Type) OR Random*” (Title) OR“Comparative Effectiveness Research” (Mesh) OR “Comparative Study” (Publication Type) Or compar*(title) NOT “Critical Care” (Mesh) OR “Child” (Mesh) OR “Infant” (Mesh) OR “Pediatrics” (Mesh) OR “Adolescent” (Mesh) Or Critical care OR child* OR infan* Or paediatr* or pediatr* OR adolescent*.
| Level of scientific evidence provided by the literature (treatment studies) | Grade recommendation |
|---|---|
| Level 1 | Established scientific evidence A |
| High power randomized comparative trials | |
| Meta-analysis of randomized controlled trials | |
| Decision analysis based on well-conducted studies | |
| Level 2 | Scientific presumption B |
| Low-power randomized comparative trials | |
| Non-randomized comparative studies well-conducted | |
| Cohort studies | |
| Level 3 | Low level of evidence C |
| Case-control Studies | |
| Level 4 (NP4) | |
| Comparative studies with considerable bias | |
| Retrospective studies | |
| Case series |
In the absence of studies, the recommendations are based on a consensus of opinions from the experts of the working group (Expert Consensus, EC), after having consulted the reading group. The absence of an evidence grade does not mean that the recommendations are not relevant and useful. It must, however, incite teams to conduct further additional studies.
3
Results
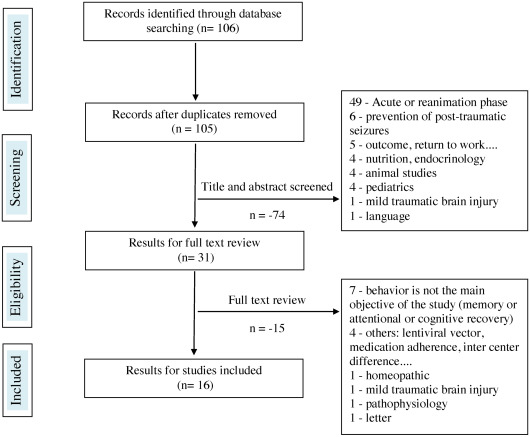
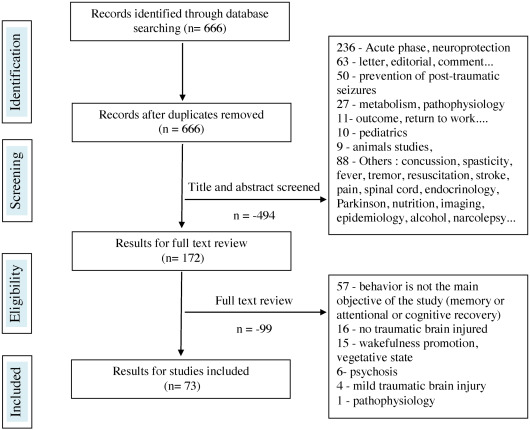
In all, 106 references were found on “drug therapy (question 4) and meta-analyses, systematic literature reviews, randomized controlled studies, comparative studies”. Out of these 16 were selected. Regarding articles focusing on all types of studies (except reviews, randomized controlled studies, comparative studies), 666 references were found, and 73 were selected.
The reasons for rejecting the studies were most often the inadequate selection of the acute phase in the ICU post-injury, including targeted articles on neuro-protection (285 rejections total), letters to the author, comments, news, editorials (56 rejections), target studies on improving cognitive performance or on a product stimulating recovery (64 rejections), absence or insufficient number of patients with TBI in the study (mixed population) for 16 rejections, or animal studies (13 rejections). The diagrams representing the selection process for the articles are illustrated in Figs. 1 and 2 . The greatest difficulty was to select articles, treating in the purest manner possible, behavioral disorders post-TBI and thus discard studies where the main objective was to improve memory, attention and cognitive performance or recovery in general. At the end, 89 references were identified and concerned 1306 people with TBI. Results are expressed by products and pharmacological class, including beta-blockers, neuroleptics, antidepressants, antiepileptics, amantadine and other drugs.
3.1
Use of beta-blocker: 33 patients included
The mechanism of action of beta-blockers on behavioral disorders remains unclear. Beyond their cardiovascular actions, beta-blockers protect against social anxiety and have been used to treat agitation or aggressiveness post-TBI ( Table 2 ).
| Article | Study description | Level of evidence | Conclusions |
|---|---|---|---|
| Brooke et al., 1992 | Randomized propranolol versus placebo study in 21 severe TBI agitated. Study of 18 months, starting dose 60 mg/day to 420 mg/day increments of 60 mg every 3 days. Study duration: 8 weeks, the initial phase after head injury. Overt Aggression Scale | Level 1 or 2 Grade B | The intensity of agitation was significantly lower in the propranolol group ( P < 0.05). No significant difference in the number of agitation episode. Reduced use of restraint measure in the treated group. Absence of cognitive effect or interaction with other treatments Limits: fairly small population, 11 patients/10 placebo |
| Greendyke and Kanter, 1986 | Randomized double-blind placebo crossover study versus propranolol, in 10 patients with organic brain disease. Beginning 80 mg/day to 520 mg/d. 4 of 9 patients are traumatic brain injured. Subjects 25 to 75 years, inclusion of 1 to 30 years later | Level 4 Grade C | Reduction of aggressive behavior ( P < 0.05) with no apparent sedative effect. Hypotension or bradycardia in 7/9 patients Limits: population heterogeneity in age, time of inclusion and causes of the brain damage No evaluation scale |
| Greendyke et al., 1986 | Randomized double-blind placebo crossover study versus pindolol, 11 patients with organic brain disease. Impulsive behavior, explosive. Subjects “severely demented”, aging 28 to 76, 5/11 patients of TBI | Level 4 Grade C | Significant decrease in the number of crisis ( P < 0.05). Optimal response dose: 40 to 60 mg/day Limits: population heterogeneity in age, time of inclusion and causes of the brain damage. No evaluation scale |
| Greendyke, 1989 | Randomized double-blind placebo crossover versus pindolol, 13 patients with organic brain disease. 3/13 are head injuries. Overt Aggression Scale. Pindolol 20 mg/twice daily | Level 4 Grade C | 8 of 13 patients improved without a statistically significant difference. Pindolol seems to decrease the verbal and physical aggression and improve quality of life of these patients Limits: population heterogeneity in age, time of inclusion and causes of the brain damage |
Regarding the treatment of agitation or aggressiveness after TBI, four studies, albeit older ones, are regularly listed . No recent studies exist. Regarding high doses of propranolol (up to 420 mg/day), the study of Brooke et al. conducted on 21 patients showed its efficacy on episodes of post-traumatic aggressiveness, with reduced intensity for the most severe episodes, yet no significant changes were noted for the frequency of these episodes (level 1 to 2, grade B).
For the other three studies, populations are heterogeneous. The study by Greendyke et al. included 9 patients treated by propranolol but only 4 of them had TBI (level 4, grade C). For pindolol , the populations studied included respectively 5/11 and 3/13 patients with TBI (level 4, grade C) Pindolol doses used varied between 20 to 100 mg/day. Adverse effects to the administration of beta-blockers are low blood pressure and bradycardia . Finally, a report of 2 cases points to metoprolol (level 4) but concerned stroke patients, not TBI patients.
In France, the use of beta-blockers in this prescription context (agitation aggressiveness) is outside of the marketing authorization (MA) delivered. The recommended usual doses of propranolol do not exceed 320 mg/day in cardiology.
The efficacy of this product is validated by the prescription habits of US experts . In the population, often quite young, of patients with TBI, this prescription is well tolerated and can represent an alternative to the prescription of psychotropic drugs. There seems to be an efficacy on impulsivity. In clinical practice, doses of 40 to 80 mg in one or two takes are often quite effective. No study was found regarding the treatment of anxiety by beta-blockers after TBI.
Beta-blockers do not have a market authorization in the care management of agitation and/or aggressiveness and/or irritability but the analysis of the literature shows that, in certain cases, they can improve these disorders. Their prescription in this indication must be evaluated according to each individual case and based on the criteria associated with treatments prescribed without MA on top of precautions of use related to this therapeutic class. The use of propranolol in the treatment of agitation and/or aggressiveness and/or irritability is proposed at the dosage of 40 to 80 mg per day even if certain studies have reported an effect with higher doses. Just like in patients with no TBI, it is recommended to start the treatment progressively and it is mandatory to wean from beta-blockers progressively because of the coronary risk. It is preferable to perform an ECG before starting a beta-blocker treatment (Experts consensus [EC]).
3.2
Use of neuroleptics and antipsychotic drugs: 52 patients included.
3.2.1
Generalities
Two reviews hardly reported the use of neuroleptics whereas other authors report their frequent use in behavioral disorders post-TBI ( Table 3 ). Review articles underlined the lack of a strong methodology and insufficient level of evidence.
| Article | Study description | Level of proof | Conclusions |
|---|---|---|---|
| Kim and Bijlani, 2006 | Quetiapine, 25 to 300 mg to 800 mg, a 6-week open study prospectively for 7 patients. Treatment of aggressiveness after TBI. Any severity. Overt Aggression Scale (OAS) and Clinical Global Impression (CGI) | Level 3 Grade C | Good efficacy and tolerance of the product Reducing irritability and aggression with improved cognitive functioning Akathisia in one subject |
| Michals et al., 1993 | Clozapine. 9 patients series with psychotic symptoms or aggression access refractory to others treatments | Level 3 Grade C | Clozapine is useful in the treatment of psychosis and aggressive behavior (partial improvement of strangeness, agitation, hallucinations), despite side effects (2 seizures/9 patients) |
| Noé et al., 2007 | Ziprasidone, 20 à 80 mg/day, 5 cases reports, management of behavioral disorders of patients with severe traumatic brain injury (TBI) during the period of post-traumatic amnesia (PTA). Agitated Behaviour Scale (ABS) | Level 3 Grade C | Efficacy of ziprasidone in controlling agitation during the PTA period. Despite the small size of the sample, ziprasidone reduced symptoms of agitation quickly and with good tolerability, safety and no side effects |
| Rao et al., 1985 | Open controlled prospective study in 26 agitated patients after severe TBI. A group of 11 required haloperidol. Measure of the length of coma, duration, of post-traumatic amnesia duration (PTA), functional status, CT-scan results | Level 3 Grade C | No significant difference in demographics, length of coma and rehabilitation success. The duration of PTA is significantly longer in the group treated with haloperidol, P < 0.05 Limits: weak statistical numbers (11 subjects) |
| Stanislav, 1997 | Case study involving 3 subjects after head injury. Evaluation of functioning differences before, during and after discontinuation of antipsychotic drugs | Level 4 Grade C | There is a cognitive improvement of people after stopping neuroleptics Limits: 3 subjects only, methodological bias |
| Umansky and Geller, 2000 | Case report. Olanzapine (20 mg/day) for psychotic manifestations following a second severe closed-head injury | Level 3 Grade C | After 6 months of treatment the individual no longer heard persecutory voices, had non delusional symptoms or rage outbursts and exhibited improvements in mood, behavior and follow up compliance |
| Viana Bde et al., 2010 | Case report. Olanzapine, 10 mg/day psychotic disorders after craniocerebral gunshot wound. Delusions religious persecution, auditory hallucinations | Level 3 Grade C | Progressive significant regression after a month of psychotic symptoms with the possibility to initiate a rehabilitation program |
According to the study conducted among expert healthcare professionals neuroleptics are not one of the 5 drugs most used to treat agitation post-TBI. Haloperidol or risperidone is used by non-experts. There are no standards or consensus regarding the use of neuroleptics. A greater sensitivity to their adverse effects post-TBI was described. The use of olanzapine was suggested .
3.2.2
Adverse effects of neuroleptics after TBI
3.2.2.1
Neuroleptic malignant syndrome (NMS)
There seems to be a greater exposure to the risk of neuroleptic malignant syndrome (NMS) post-TBI . This syndrome is reported as a rare occurrence. The use of haloperidol was most often reported for the 10 cases published . It concerned young adults. A case was reported under risperidone . For Levy et al., NMS is not dose-dependent. Conversely, the review of 9 cases by Bellamy et al. reported the exact opposite with the onset of neuroleptic malignant syndrome under high doses of haloperidol. The most recent review , underlining the challenges in making the diagnosis, reported that in 90% of the cases, the first symptoms appeared on average 10 days after the beginning of the neuroleptic treatment. A hypodopaminergic state of the brain related to the trauma was brought up.
3.2.2.2
Potentially noxious effects of neuroleptics on recovery abilities
On animal models (rat) haloperidol was reported to affect recovery after damage to motor cortical areas and interacts with the neuronal recovery process. For Wilson et al, multiple administrations olanzapine would not impact cognitive functions, conversely to haloperidol. Another study showed a deterioration of motor and cognitive performances after chronic administration of high doses of haloperidol or risperidone. Unique or repeated low doses of haloperidol or risperidone seem safe vs. continuous high doses negatively affecting recovery (animal study). A year later, the same team showed that chronic administrations (during 19 days) of low or higher doses of haloperidol or risperidone prevented motor and cognitive recovery. This effect did not seem related to the sedative properties , but rather to the alteration of neurotransmitter systems.
In humans, a study conducted on 11 people treated by haloperidol after TBI vs. controls reported a significant increase of the duration of the post-traumatic amnesia in the treated group (level 2). Another study , described a cognitive improvement of patients after having stopped taking neuroleptics, but the study only included 3 subjects and had potential biases (level 4). Thus, we have several studies describing the negative interference of neuroleptic treatments with the biological and brain plasticity process, essence of the recovery project . However, these studies do not bring forward real evidence on a scientific level.
3.2.2.3
Neuroleptics and apathy post-TBI
No study was found using neuroleptics post-TBI to treat apathy. Review studies on this symptom in other neurological or psychiatric pathologies (low level of evidence) reported an improvement of apathy in schizophrenia, major and non-psychotic depression and Alzheimer’s disease with second-generation neuroleptics .
3.2.2.4
Practical advice for the use of neuroleptics post-TBI
In the absence of consensus proposals, the combined data of the different studies analyzed can define practical rules to the specific use of neuroleptics post-TBI.
First do no harm, do not prescribe neuroleptics. If possible wait or propose another non-pharmacological approach (institutional and/or psychotherapeutic) or an alternative to neuroleptics: e.g. beta-blockers, mood stabilizers. In the absence of objective agitation or aggressiveness, it is recommended to limit the use of neuroleptics to cases when other care management therapeutics have failed. Regardless of the pharmacological approach taken, it is necessary to view the problematic of the efficacy on one symptom in the context of individual neurological recovery. Neuroleptics might have a negative impact on neuronal plasticity, thus having a reverse effect on the rehabilitation’s main objective.
In case of emergency and acute aggressiveness the prescription of neuroleptics can be envisioned in the absence of contraindications.
Outside of the acute crisis or emergency situation, to start a neuroleptic treatment, it is preferable to:
- •
start with a small dosage go slowly and progressively when increasing the dosage continuously reassess the clinical presentation;
- •
one single product at a time (monotherapy);
- •
be careful of interactions between products;
- •
monitor the epileptogenic threshold;
- •
the experience of TBI consequences, from awakening to the chronic phase can alert prescribing physicians on the high sensitivity of TBI patients to sedative drugs, the rule being the “minimum effective dose”.
Prefer the use of an atypical neuroleptic (2nd generation) that has less side effects , especially less extrapyramidal side effects. Antipsychotics have a better benefit/risk ratio.
Several authors estimate that one should not use neuroleptics on the long term to treat aggressiveness after TBI except in case of prior psychiatric disease . The premorbid personality and psychiatric history can guide the therapeutic choices.
3.2.2.5
Data per product (second generation, atypical neuroleptics)
Some studies ( Table 3 ) with a low level of evidence (level 3 to 4) concerned case reports reporting the effectiveness of ziprasidone in agitation , quetiapine and olanzapine after aggressiveness or post-traumatic psychosis or clozapine in this symptom with hematologic risks . Another case report (severe TBI) showed the efficacy of olanzapine on hallucinations .
There is no sufficient evidence regarding the efficacy of neuroleptics in the treatment of behavioral disorders such as irritability, aggressiveness or apathy post-TBI.
Observation: There are no “new” nor specific side effects related to the use of neuroleptics post-TBI. However, there are several specificities that need to be accounted for:
- •
greater exposure to neuroleptic malignant syndrome;
- •
sedative effect that could increase the risk of falls or dysphagia;
- •
potential noxious effects on brain plasticity and thus on the recovery potential.
In case of emergency or acute agitation and aggressiveness crisis, the prescription of a neuroleptic can only be envisioned in the absence of contraindications, to obtain a quick sedation in order to protect the patient from self-harm, protect his/her closed ones or the healthcare team. Loxapine (Loxapac * ) has a marketing authorization in its injectable form for treating “cases of agitation, aggressiveness and anxiety associated with psychotic disorders, or certain personality disorders” (EC). The long-term use of neuroleptics for the treatment of behavioral disorders in patients with TBI must be avoided due to side effects, except in case of prior psychiatric disease (EC). In the absence of therapeutic alternatives, the use of neuroleptic must respect the usual prescription guidelines of this therapeutic class. Furthermore, in patients with TBI, it is recommended to abide by prescription guidelines common to the different therapeutic agents (see above). More specifically, regarding neuroleptics it is essential to take additional precautions (EC): (i) take into account the epileptogenic risk, since the threshold might be lower than usual, (ii) use preferentially an atypical neuroleptic (2nd generation) because it leads to less side effects, especially less extrapyramidal side effects, (iii) be aware of the cardiovascular risks.
3.3
Use of antidepressants, 348 patients included
The scientific literature reporting the effects of antidepressants after TBI is quite scarce ( Table 4 ). The effect of these products on mood and behavior has rarely been studied. The specific action of antidepressants on behavioral disorders remains to be validated.
| Article | Study description | Level of evidence | Conclusions |
|---|---|---|---|
| Ashman et al., 2009 | Randomized 10 weeks double-blind sertraline (early 25 mg up to 200 mg/day) versus placebo in 52 volunteers after TBI. Using Hamilton Rating Scale for Depression (HAM-D). Chronic phase: 17 ± 14 years after the accident | Level 1 Grade A | No significant difference between the two groups on depression measures, anxiety and quality of life. There is an improvement of 3 scores (depression, anxiety, quality of life) in the 2 groups (59% sertraline group, 32% placebo group) Limits: heterogeneous population |
| Dinan and Mobayed, 1992 | Cohort 6-week study of amitriptyline (up to 250 mg), 13 mild TBI with depression matched with 13 depressed patients without TBI. Using modified Hamilton Rating Scale for Depression | Level 2 Grade C | Only 4 patients showed marked improvement in MTBI group versus 11 in depressed patients without TBI. Depression following MTBI is relatively resistant to tricyclic antidepressants treatment. Amitriptyline is effective in some TBI cases |
| Fann et al., 2000 | Non-randomized single-blind 8-week study, sertraline 25–150 mg/day versus placebo. 15 patients after Mild TBI in major depression, on average 10.6 months later. Modified Hamilton Rating Scale and DSM-III criteria | Level 3 Grade C | Statistically significant improvement in depression, psychological disorder, hatred, aggression and post concussive symptoms Limits: no randomized group and single-blind |
| Horsfield et al., 2000 | There are data that show that fluoxetine is able of neuronal remodeling Open pilot study, fluoxetine (20–60 mg/day) administered to a group of five head injury with moderate or non-depressed depression. Initial cognitive tests and after eight months | Level 4 Grade C | Fluoxetine improves mood, and performance on the Trail Making Test Part A, and WAIS-III Although fluoxetine has had beneficial effects on certain measures of cognition, more work is needed to link these improvements with the neuronal remodeling |
| Jackson et al., 1985 | Amitriptyline case study. 32-year-old man, severe agitation after TBI and injury to the frontal lobes. Failure of others treatments | Level 3 Grade C | Efficiency in 2 weeks, with cessation of anger, agitation, without significant cognitive side effects. Respect for cognitive performance |
| Kanetani et al., 2003 | Milnacipran (30 to 150 mg/d) reuptake inhibitor of serotonin and norepinephrine (SNRIs), to treat depression after mild to moderate TBI. Open study of six weeks for 10 patients (4 men and 6 women) from 28 to 74 years. DSM-IV Hamilton Scale (HAM-D). Cognitive status was assessed by the mini mental test (MMSE) | Level 4 Grade C | On the basis of a HAM-D decrease more than 50% response rate was 66.7%, the remission rate of 44.4%. Significantly greater improvement in cognitive functions in patients. Milnacipran is a safe and effective drug for depression after ECT and could be used as first line |
| Kant et al., 1998 | Open trial without control group, sertraline 50–200 mg/day. Using Modified OAS scale. 13 irritable and aggressive patients after severe TBI ( n = 2), moderate ( n = 6) or mild ( n = 5). Study 8 weeks, irritability and depression are evaluated every 2 weeks | Level 4 Grade C | Significant reduction in irritability, aggression and critical access, no effect on depressive symptoms after 8 weeks. The SRI are useful for the treatment of irritability and aggression after TBI, the improvement is not due to the improvement of depressive symptoms Limits: lack of control group. Alcohol use for 2 patients and drug use for 1 |
| Lee et al., 2005 | Randomized 4-week double-blind placebo study vs. sertraline or methylphenidate and Neuropsychiatric sequelae after mild to moderate TBI. Thirty patients, methylphenidate (5 mg/day to 20 mg/day), sertraline (25 mg to 100 mg/day) placebo. Before and after 4 weeks, 13 tests on depression, quality of life, cognitive performance, sleep | Level 2 Grade B | Methylphenidate and sertraline had similar effects on depressive symptoms. Methylphenidate seems superior in improving cognitive function and maintaining daytime alertness. Methylphenidate also has better tolerance than sertraline |
| Meythaler et al., 2001 | Prospective randomized study sertraline (100 mg/day) versus placebo in improving wakefulness and alertness after TBI in initial rehabilitation phase. 11 patients. ABS, Agitated Behavior Scale, Orientation Log, Galveston Orientation and Amnesia Test (GOAT) | Level 2 Grade B | Placebo and medication have the same levels of improvement for the 3 tests. No significant differences between the two groups No negative effect on recovery or side effects. Limits: small sample – Expected effects from the first week, short essay (2 weeks). Significant bias |
| Mysiw et al., 1998 | Retrospective Amitriptyline study, 20 agitated patients with post-traumatic amnesia stage after TBI. Orientation Group Monitoring System | Level 4 Grade C | Significant improvement of the agitation for 12/17 patients within 7 days after product initiation No interaction with cognitive recovery |
| Newburn et al., 1999 | Retrospective case series of Moclobemide (450–600 mg/day), 26 patients with TBI and depression diagnosis Hamilton Depression score (HAM-D) | Level 4 Grade C | Reduction of the HAM-D 81%. Fast action, 17 responders J3. Reducing irritability scores by 57% and 39% for mental pain. Moclobemide may be an effective treatment for major depression after ECT, controlled studies are lacking |
| Perino et al., 2001 | Open prospective study of 12 weeks of citalopram (20 mg/day) and carbamazepine (CBZ) (600 mg/day) in 20 depressed patients after severe TBI. MIF, Brief Psychiatric Rating Scale (BPRS) and Clinical Global Impression Scale (CGI) | Level 4 Grade C | (a) Citalopram combined with CBZ reduces depression and behavioral disorders after TBI, P < 0.05. (b) These disorders must be taken care of as soon as possible during the rehabilitation period. Since combination therapy was used, it is not possible to determine whether one or the other drug was primarily responsible of the improvement |
| Rapoport et al., 2008 | Open study citalopram (20 mg to 50 mg/day) for 6 weeks and 10 weeks in 54 patients with major depression following mild to moderate TBI Hamilton Depression Scale (HAM-D) | Level 4 Grade C | The response rate is comparable to the results of the testing of patients with major depression but has not had TBI |
| Rapoport et al., 1999 | Randomized double-blind study citalopram (20–50 mg/day) versus placebo in 65 patients with major depression. Is continuous treatment with citalopram prevents a relapse of depression after TBI? Remission Score ≤ 7 for Hamilton Rating Scale. Relapse if HAM ≥ 16 | Level 2 Grade B | 11 relapses of depression (52.4%) Relative risk of recurrence of depression after TBI is 50%. It is better to stop the treatment and secondarily take citalopram for depression relapse Limits: 10 versus 11 placebo subjects citalopram, 3 outputs study including 1 for diarrhea |
| Slaughter et al., 1999 | Two cases of Klüver-Bucy syndrome after severe TBI posing care problem | Level 3 Grade C | Benefit of the SRI to treat Klüver-Bucy Sd after TBI |
| Teng et al., 2001 | Case study. An agitated man after severe TBI in the recovery phase. Failure of others products. Treatment with Bupropion (Zyban) | Level 3 Grade C | The use of Zyban (150 mg/day) is not discussed in any other study. In this case, it is the only treatment that could significantly reduce agitation and improve cognitive function |
| Wroblewski et al., 1996 | Randomized, placebo-controlled prospective crossover study about desipramine (150–300 mg/j). 10 individuals with severe TBI and depression. Product not commercialized in France | Level 3 Grade C | Of the 7 patients who completed the study, 6 improved on desipramine treatment. 2 study outputs: a seizure, a manic turn Significant methodological flaws and small sample size limited the strength of the findings |
No argument in favor or against the use of antidepressants in apathy was found. Regarding agitation and aggressiveness, the level of evidence is low and contrasts with the relatively frequent use of antidepressants. For depression itself, their usefulness is more frequently demonstrated.
3.3.1
Agitation aggressiveness
Several arguments support the use of antidepressants to treat agitation and aggressiveness. Animal studies have shown that serotonin levels were negatively correlated to aggressiveness . Dopaminergic and noradrenergic circuits, greatly involved in executive functions , are commonly disrupted after TBI, which could promote the onset of behavioral disorders .
Conversely, antidepressants can have adverse effects and increase confusion, sleepiness (at all stages of the care management) and/or anxiety, induce nausea, anticholinergic effects for some and increase the risk of suicidal attempts.
Based on a survey conducted on US medical specialists , antidepressants are one of the 5 most frequently used drugs to treat agitation in patients with TBI.
Regarding agitation and aggressiveness, articles are scarce. The only study with a control group, sertraline (100 mg/d, 11 patients) vs. placebo, did not show significant effects on agitation (level 2). But the number of patients was low and the study duration was only 15 days . According to Lombard et al. the antidepressant effect cannot be reached before ≥ 2 weeks. These authors consider that Selective Serotonin Reuptake Inhibitors (SSRIs) have a non-acceptable delay of action for the control of acute agitation. A series of 13 patients with TBI under sertraline 200 mg/d without a group control, unveiled a significant effect on irritability and aggressiveness (level 3), whereas no effect was noted on depression symptoms after 8 weeks of treatment. In two patients presenting with Klüver-Bucy syndrome (agitation, hyperorality, hypersexuality) after severe TBI, the use of sertraline up to 150 mg/d was followed by an improvement in a few days of disorders that were resistant to other drugs (level 3). For one of these two cases, the association with a neuroleptic seemed to have increased the effect of sertraline.
Amitriptyline is not commonly used in agitation after TBI . It is considered by some authors as the pharmacological agent of choice in the treatment of behavioral disorders after frontal brain damage . A retrospective study comparing 20 agitated patients treated with amitriptyline to a control group of 38 non agitated patients found for patients in the stage of post-traumatic amnesia a great efficacy on agitation for 12 patients out of 17 after 7 days of treatment (beginning at 25 mg/d up to 150 mg/d, level 3). For a 32-year-old agitated patient with TBI and bifrontal damage, amitriptyline was reported to be effective in 2 weeks on anger bursts with an improvement of attention (level 3).
Interestingly, one should note the effectiveness of bupropion (Zyban ® ) at quite high doses (150 mg/d) in a 20-year-old woman described as very agitated after the failure of loxapine, haloperidol, propranolol, methylphenidate, diazepam and paroxetine (level 3). This treatment cannot be recommended today based on current scientific knowledge.
SSRIs present less side effects and a profile of side effect different from tricyclics that can generate sedation, as well as other side effects such as cardiologic ones, anticholinergics with constipation and urine retention, as well as lowering the epileptogenic threshold . Neither SSRIs nor tricyclic antidepressants have a marketing authorization in France for agitation or aggressiveness.
3.3.2
Depression
The literature reviews by Alderfer et al., 2005 and Fann et al., 2009 reported the best level of evidence for noradrenergic and specific serotonergic antidepressants (NaSSAs) for the treatment of depression. A randomized, double-blind vs. placebo study after TBI with sertraline (25 to 200 mg/day) validated after 10 weeks of treatment an improvement in mood, quality of life and anxiety (level 2).
Other studies already pointed out this result with sertraline (level 4). A study on fluoxetine (Prozac ® ), to look for cognitive effects and impact on brain plasticity , found an improvement on mood as well as memory and attention performances (level 4). A work on milnacipran (Ixel ® , 30 to 150 mg/day) after light to moderate TBI for a 6-week duration, described an improvement of depression and cognitive performances (level 4). This antidepressant is a selective serotonin and norepinephrine reuptake inhibitor (SNRI). The use of moclobemide (Moclamine ® ) post-TBI in an open study showed the good efficacy of the product (level 4). Regarding citalopram (Seropram ® ), a 2001 study described an 81% response rate after TBI (level 4). An open study on 54 patients with light to moderate TBI treated with citalopram , reported a 46% response rate with a remission at 26.9% after 10 weeks of treatment. These results are comparable to results of patients suffering from major depression, without TBI (level 4). A randomized, double-blind study citalopram vs. placebo reported the limits of pursuing the treatment to avoid depression recurrence. It is preferable to stop the treatment and secondarily start again on citalopram in case of recurrence (level 2).
Regarding tricyclic antidepressants, a randomized, crossover study focused on desipramine (not marketed in France) vs. placebo . The samples were small (6 patients with TBI validated the study), (level 3). For amitriptyline, a study on 13 patients with TBI compared to 13 patients with depression showed a 4/13 response rate in the group of patients with TBI vs. 11/12 in the group without TBI . Authors concluded that depression after TBI was relatively resistant to treatment by tricyclic antidepressants (level 4).
Just like the treatment for aggressiveness, the treatment for depression must seek the best benefit/risk ratio, which tends to prefer SSRIs rather than tricyclic antidepressants as first line treatment.
3.3.3
Apathy
For apathy, no specific study was found regarding this specific symptom sometimes identified by certain authors in cognitive functions. Apathy is often symptomatic of a depressive state , antidepressants could be envisioned as an initial treatment. The effect on awakening could also have a positive impact.
3.3.4
Other disorders
The use of paroxetine and citalopram seem interesting in the treatment of pathological cries even if results must be validated in further studies on larger samples (level 3).
Antidepressants are mainly used to treat depression according to the usual recommendations and in the framework of their MA. In the absence of contraindications, they can be used after TBI (EC). The prescription of antidepressants in the context of the treatment of depression in all the stages of its progression, must abide by the guidelines for clinical practice published by the ANAES in 2002: “management of an isolated depressive episode in adult outpatients” (EC). Specifically, it was indicated that the choice of an antidepressant should preferentially be based on specific criteria:
- •
therapeutic use of lateral effects (for example, looking for sedation, anxiolytic effect or stimulation);
- •
the preferential indication of a therapeutic class in some psychiatric comorbidities, for example Selective Serotonin Reuptake Inhibitors (SSRIs) and specifically sertraline for obsessive compulsive disorders;
- •
the mixed effect SSRI + adrenergic (SSNRI) on cognitive disorders for example milnacipran (Ixel ® ) at the dose of 30 to 150 mg (EC).
Observation: Antidepressants can be effective in an indirect manner on agitation and aggressiveness when considering that depression or anxiety are promoting factors. Amitriptyline showed a certain efficacy on agitation from the dose of 25 mg per day. Paroxetine and citalopram can improve pathological cries of certain patients after TBI. SSRIs could have a positive impact on brain plasticity and thus on the recovery potential. Sertraline showed some efficacy on agitation, aggressiveness and irritability with doses going up to 200 mg per day. An improvement of Klüver-Bucy Syndrome (agitation, hyperorality, hypersexuality) after severe TBI, was reported with sertraline up to 150 mg/d. The positive effect might have been majored by associating sertraline with a neuroleptic. In the absence of specific indications, it is recommended to choose the best tolerated antidepressant, the less dangerous one in case of massive absorption, and the simplest to prescribe at an effective dose. SSRIs, SSNRIs and other non-tricyclic, non-MAOI antidepressant drugs are the ones most adapted to these criteria. During the awakening period, tricyclic antidepressants and especially amitriptyline at a low dose can also be proposed to treat agitation (EC). Antidepressants do not have a MA in France to treat agitation and/or aggressiveness, so the prescription of these molecules should be evaluated for each individual case according to the criteria associated with treatments prescribed outside of a MA on top of the usual recommendations (EC).
3.4
Antiepileptic mood stabilizers and other antiepileptic drugs, 555 patients included
3.4.1
Carbamazepine (CBZ), valproate (VPA) and phenytoin
Carbamazepine (CBZ) and valproate (VPA) are antiepileptic agents commonly used as mood stabilizers to treat agitation after TBI ( Table 5 ). VPA has the reputation of having less side effects . In France VPA and CBZ do not have a marketing authorization for agitation, irritability or aggressiveness.
| Article | Description of study | Level of proof | Conclusions |
|---|---|---|---|
| Azouvi et al., 1999 | Prospective open trial of carbamazepine (400–800 mg/day, 8 weeks) on agitation and aggressive behavior in 10 patients with a severe TBI Agitated Behavior Scale (ABS), Katz Adjustment Scale (KAS) for social functioning, Mini Mental Status Examination (MMSE) | Level 3 Grade C | Statistically significant improvement ( P < 0.05) on irritability and disinhibition in particular, the ABS and KAS score. No global cognitive change found on MMSE scores Carbamazepine might help to reduce agitation after TBI |
| Chahine and Chemali, 2006 | A retrospective study about 4 young patients with pathological laughter and/or pathological crying following TBI. A lamotrigine treatment | Level 3 Grade C | The four patients were successfully treated with lamotrigine |
| Chatham Showalter, 1996 | Use of carbamazepine for 7 combative patients with multiple trauma including TBI | Level 4 Grade C | This cohort had a clinical decrease in combativeness within 4 days after carbamazepine |
| Chatham Showalter and Kimmel, 2000 | Retrospective study for 22 months in a rehabilitation unit of a group of 29 subjects for Divalproex under agitation. 23 TBI patients, 5 strokes, 1 vasculitis | Level 4 Grade C | For 26 of the 29 patients, effective in 7 days at a dose of 1250 mg/day. An effective alternative to antipsychotics and benzodiazepines against impulsivity and disinhibition after brain injury |
| Dikmen et al., 2000 | Randomized double-blind study, Valproate (VPA) versus. Phenytoin, comparison between prevention of epilepsy and neuropsychological effects at 1 and 6 months. 279 adults randomized within 24 hours of the injury, battery of neuropsychological measures at 1, 6 and 12 months | Level 2 Grade B | No negative nor positive neuropsychological effects of VPA. VPA seems to have a benign neuropsychological effects profile. However, for this study, the VPA does not prevent post-traumatic crises No placebo group |
| Gościński et al., 1997 | 4 patients with post-traumatic lesions localized bitemporally developed Kluver-Bucy syndrome Treatment with carbamazepine | Level 4 Grade C | Several symptoms responded dramatically to carbamazepine. A useful agent in treatment of this unusual syndrome |
| Kim and Humaran, 2002 | Retrospective study, 11 patients after TBI, Divalproex alone or with other psychotropic proposed on neurobehavioral symptoms | Level 4 Grade C | Divaproex is well-supported after TBI Limits: heterogeneity of patients and treatments, 3 bipolar, 2 psychotics, 2 personality changes |
| Mattes, 2005 | Randomized double-blind oxcarbazepine (1200–2400 mg/day) versus placebo for 10 weeks. 48 aggressive patients, multiple medical causes Global Overt Aggression rating, Overt Aggression Scale-Modified | Level 2 Grade B | Consistent evidence in favor of oxcarbazepine, compared to placebo ( P = 0.012). Oxcarbazepine seems to bring benefit to aggressive adults regardless of the cause of the aggression. Limits: Mixture of pathologies. 9 patients discontinued due to adverse effects, 45 completing the study at least 4 weeks |
| Mattes, 2008 | Randomized double-blind levetiracetam (3000 mg/day) versus placebo for 10 weeks, in aggressive patients (no TBI study) Overt Aggression Scale-Modified | Level 2 Grade B | Of 40 patients (20 in each treatment group), 34 completed at least 4 weeks of treatment with double-blind medication. There was no overall statistical evidence of levetiracetam benefit |
| Perino et al., 2001 | Open prospective study of 12 weeks of citalopram (20 mg/day) and carbamazepine (CBZ) (600 mg/day) in 20 depressed patients after severe TBI. MIF, Brief Psychiatric Rating Scale (BPRS) and Clinical Global Impression Scale (CGI) | Level 4 Grade C | (a) Citalopram combined with CBZ reduces depression and behavioral disorders after TBI, P < 0.05. (b) These disorders must be taken care of as soon as possible during the rehabilitation period. Since combination therapy was used, it is not possible to determine whether one or the other drug was primarily responsible for the improvement |
| Pachet et al., 2003 | Single case study. Effectiveness of lamotrigine to treat aggressive and agitated behavior in a 40-year-old male who sustained a severe TBI. | Level 3 Grade C | A substantial decrease in behavior problematic and a significant improvement in neurobehavioral functioning were observed after lamotrigine treatment |
| Smith et al., 1994 | Double-blind versus placebo study, phenytoin and carbamazepine (CBZ) prophylaxis of epilepsy after TBI. 40 of 64 patients receiving phenytoin and 42 of 127 receiving CBZ from 6 to 44 months. Neuropsychological tests twice during a 4-week baseline period, at the end of a 4- to 5-week period of continued drug treatment or placebo, and after 4 weeks of not receiving medication. Attention and psychomotor tests, speed, memory, verbal fluency, emotional state | Level 2 Grade B | Patients in groups CBZ and phenytoin had a significant improvement ( P < 0.01) on several measures of motor performance and executive speed when stopping medication. Both treatments appear to have negative effects on cognitive performance, particularly the speed of psychomotor tasks |
| Wroblewski et al., 1997 | Single case study. Effectiveness of valproate (VPA) on destructive and aggressive behaviors in 5 patients after acquired brain injury (4 TBI) | Level 3 Grade C | For these 5 cases, effectiveness in one to two weeks where other treatments have failed. Fewer side effects, VPA complies potential participation in rehabilitation |
Studies describing cognitive side effects of VPA or CBZ after TBI are contradictory. A review on phenytoin, CBZ and VPA from 1991 reported that these three medicines seemed to exert a negative effect on cognitive and motor functions. These disorders get worse with serum levels . In this randomized, double-blind CBZ and phenytoin vs. placebo study, authors found a negative impact on cognitive performance for these two products (level 2). Conversely, a prospective, randomized, double-blind study comparing 279 patients with TBI receiving valproate or phenytoin, showed that the valproate treatment did not have any negative or positive neuropsychological effects (level 1).
According to the survey conducted on US physicians , CBZ is listed as the primary treatment to treat agitation after TBI. The work conducted 10 years later on agitation, anger and irritability reported sodium valproate as the main treatment.
Regarding treatment of agitation and aggressiveness by VPA, a prospective study on 29 patients, including 23 who presented with TBI described an improvement in 26 patients . The efficacy was described as rapid, with doses comprised between 1000 and 1800 mg per day (mean 1200 mg) of sodium valproate (level 3).
For more heterogeneous disorders (bipolar, psychotic) another retrospective study reports a good efficacy and good tolerance for 11 patients after TBI (level 4). Another series of 5 subjects (4 TBI/5) reported the efficacy of VPA where other treatments had failed (level 3).
Regarding the use of carbamazepine (400 to 800 mg/day) in 10 patients agitated with anger bursts in a prospective open 8-week study reports a good efficacy (level 3) with no negative cognitive effects. A study on 7 aggressive patients with TBI reported the efficacy of carbamazepine (400 to 900 mg/day) in the 4 days following the beginning of treatments, and in four cases CBZ was associated with haloperidol, allowing the decrease of neuroleptics intake (level 4). A Polish case series of 4 subjects with Klüver-Bucy Syndrome after post-traumatic bilateral temporal injury, showed a very good efficacy of CBZ (level 4). Finally, Perino et al. reported the efficacy of the citalopram/CBZ (600 mg/d) combination in 20 subjects with major depression and behavioral disorders, among the 37 subjects of a prospective open study (level 4).
3.4.2
Oxcarbazepine (OXC) (Trileptal ® )
There is no study on behavioral disorders treated by OXC after TBI. OXC appears to have an efficacy similar to CBZ and less side effects in children, adolescents and adults suffering from partial seizures . A randomized, double-blind OXC vs. placebo study (24 people per group) brought convincing evidence of the superiority of OXC for the treatment of aggressiveness, independently of medical causes (level 2). The same author published a focus on oxcarbazepine in the treatment of aggressiveness in prison . The search for the slightest side effect and an efficacy, even a partially proven one, guides towards using anti-seizure drugs as first line treatment for aggressiveness. Nowadays, a wider use of OXC is being discussed.
3.4.3
Lamotrigine (Lamictal ® )
Only one case study was found, concerning a 40-year-old patient with severe TBI treated successfully for his aggressiveness by lamotrigine (level 3). There are 4 published cases of laughs and spasmodic cries treated successfully by lamotrigine (level 3).
3.4.4
Gabapentin (Neurontin ® )
No study was found on gabapentin and neurobehavioral disorders post-TBI. There is one published case of aggravation of agitation after TBI associated with the prescription of gabapentin for neuropathic pain .
3.4.5
Levetiracetam (Keppra ® )
Another study on aggressive patients without TBI seems to underline the lack of efficacy of levetiracetam on this symptom (level 2).
The use of antiepileptic agents for epilepsy treatment or prophylaxis after TBI suggests an efficacy of carbamazepine, sodium divalproate and sodium valproate in the treatment of agitation and aggressiveness. Carbamazepine (CBZ) (Tegretol ® ) and sodium valproate (VPA) (Depakine ® ) are recommended as first line treatment after TBI to treat agitation, aggressiveness, anger and irritability especially in the presence of mood swings. Nevertheless, the use of antiepileptic drugs having a mood stabilizing effect, has not been validated by a marketing authorization to treat agitation, aggressiveness, anger and irritability and the prescription of these molecules must be evaluated for each individual case, according to the criteria associated with treatments prescribed outside a MA on top of the usual precautions of use (EC).
Observation: The efficacy of VPA is described as rapid, with a relatively standardized dose of 1250 mg of sodium divalproate. The good efficacy of CBZ was reported with a dose of 400 to 900 mg/day without negative cognitive side effects. An improvement of post-traumatic Klüver-Bucy syndrome was reported for CBZ treatment. Some authors promote the use of oxcarbazepine (OXC) (Trileptal ® ) instead of CBZ due to its efficacy on agitation and aggressiveness and a better profile of side effects. Regarding Lamotrigine (Lamictal ® ), an improvement of aggressiveness and spasmodic cries and laughs was reported in case studies. Levetiracetam (Keppra ® ) has no positive effects on aggressiveness post-TBI. It should be avoided after TBI because of the risks of behavioral and mood disorders induced by this drug agent (EC).
3.5
Benzodiazepines
According to the survey conducted on US physicians , benzodiazepines (BZD) are not used by the expert group and are firsthand used by non-experts (5th place). The similar work dating from 2007 pointed to the same results for agitation, whereas BZD were not listed for treating anger or irritability.
Regarding agitation and aggressiveness, the reviews described the potential noxious effects of benzodiazepines (BZD) after a stroke or brain damage . Some bothersome side effects were reported such as paradoxical agitation, especially in older subjects or an amnestic effect by nature, which might promote confusion in people in the post-traumatic amnesia. For some authors, BZD should only be used in emergency situations and should not be used on the long term in the treatment of agitation post-TBI . It is preferable to limit its use to situations where anxiety is the predominant symptom and for episodic agitation. One should take into account its sedative effect and its limitation with a negative impact on cognitive recovery.
An Australian meta-analysis assessed the effects of long-term use of benzodiazepines (more than 1 year) on the normal population, without TBI. The use reported the evidence of a global cognitive impact on all the variables assessed (sensory processes, memory, processing speed, attention/concentration, general intelligence, work memory, psychomotor speed, problem resolution, motor control and performances, verbal reasoning) (level 1).
There is not enough evidence on the efficacy of benzodiazepines in the treatment of agitation or aggressiveness in patients with TBI. BZD can be used in situation of emergency and should not be used on the long term in the treatment of agitation after TBI. The use will be limited to situations were anxiety is the predominant symptom and privileging a short-term use (symptomatic prescription) (EC). After TBI, the use of benzodiazepines must take into account the risk to generate or aggravate awareness, attention and/or memory disorders, potential respiratory depression, risk of a paradoxical effect on agitation, and its inhibition of brain plasticity capacities. There is also a risk of dependence and addiction (EC).
3.6
Amantadine, 295 patients included
Regarding the treatment of agitation with amantadine, US experts list this product among the 5 first ones , before beta-blockers ( Table 6 ). Amantadine is an antiparkinsonian, antiviral agent, which increases the availability of pre- and post-synaptic dopaminergic markers in the striatum. It is also a NMDA (N Methyl D Aspartate) receptor antagonists .
| Article | Description of study | Level of proof | Conclusions |
|---|---|---|---|
| Chandler et al., 1988 | Two cases of recovering brain injury patients with difficult-to-treat destructive behavior, whose agitation and aggression responded to amantadine | Level 3 Grade C | Direct-acting dopamine agonists such as amantadine may be the preferred treatment for patients with behavior problems in the acute stages of recovery from coma |
| Hammond et al., 2014 | Double-blind randomized prospective study amantadine 200 mg/day ( n = 36) versus placebo ( n = 36) more than 6 months after TBI with irritability and aggression for 28 days. Using the Inventory Neuropsychiatric irritability (NPI-I) | Level 1 Grade A | Agitation and aggression are significantly decreased ( P = 0.0016) in frequency and severity in the amantadine group No difference in the occurrence of side effects between the two groups |
| Hammond et al., 2015 | Double-blind, randomized, multicentre prospective study amantadine ( n = 75) 200 mg/day versus placebo ( n = 82) more than six months after TBI with irritability and for 60 days. J28 and J60 Evaluation. Using the Neuropsychiatric Inventory (NPI-I) and Clinical Global Impression (CGI) | Level 3 Grade C | Despite a significant improvement in the amantadine group, there is no significant difference between the two groups at D28 and D60. No difference in the occurrence of side effects between the two groups Placebo effect may have masked the detection of a drug effect (through observation) |
| Kraus et al., 1997 | Seven case series (6 with TBI, 1 with meningitis) with “frontal lobe syndrome” including impulsivity, disinhibition, poor motivation. Using amantadine, 400 mg/day. Patients received neuropsychiatric examinations and serial neuropsychological testing | Level 4 Grade C | All patients showed some degree of positive response. One had side effects that resolved upon discontinuation of drug |
| Meythaler et al., 2002 | A prospective, randomized, double-blind, amantadine (200 mg/day) versus placebo-controlled (6 weeks), crossover design. 35 subjects, who had a TBI with a Glasgow Coma Scale score of 10 or less within the first 24 hours after admission | Level 2 Grade B | Improvement in various tests, ( P < .05), in the group that received amantadine during the first 6 weeks, but there was no improvement in the second 6 weeks on placebo ( P > .05). There was a consistent trend toward a more rapid functional improvement regardless of when a patient was started on amantadine in the first 3 months after injury |
| Nickels et al., 1994 | Retrospective review of 12 subjects (10 with TBI) with brain injury who were treated with amantadine | Level 4 Grade C | Ten of the 12 subjects exhibited some improvement in cognitive and/or physical function while on amantadine. Two of the three subjects with severe agitation showed dramatic resolution of the agitation. Eight of nine low-arousal subjects displayed an increased level of responsiveness. Amantadine may play a role in neurobehavioral recovery of brain injury |
| Schneider et al., 1999 | A prospective, randomized, double-blind, amantadine versus placebo-controlled (2 weeks), crossover design. Subjects were 10 adult traumatic brain injury patients in an acute brain injury rehabilitation unit. Various cognitive tests | Levels 2–3 Grade B | No significant difference for amantadine versus placebo although patients generally improved Limits: small sample size, heterogeneous population, acute time course, and large number of dependent variables |
| Van Reekum et al., 1995 | Case study. Severe amotivation, apathy, and abulia, significantly retard rehabilitation following severe TBI. A double-blind, amantadine (300 mg/day) placebo-controlled study. Four treatment periods of 2 weeks duration | Level 4 Grade C | This one case study suggests possible benefit with amantadine for patients with amotivational syndrome after traumatic brain injury. There were no side effects |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






