Diarrheagenic Escherichia Coli
James P. Nataro
Larry K. Pickering
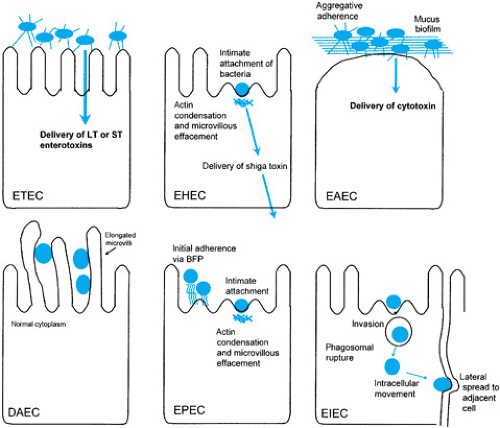
Escherichia coli is the predominant aerobic gram-negative organism of the human intestine. Whereas most E. coli isolates are harmless intestinal commensals, several highly adapted E. coli have developed the ability to cause a spectrum of human diseases. The diarrheagenic E. coli can be subdivided into six distinct categories, each having a characteristic mode of pathogenesis (Fig. 160.1), epidemiology, and clinical presentation (Table 160.1). These categories include several established
and emerging pathogens of worldwide public health importance.
and emerging pathogens of worldwide public health importance.
TABLE 160.1. CATEGORIES OF DIARRHEAGENIC ESCHERICHIA COLI | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
Diagnosing any of the diarrheagenic E. coli pathotypes can be challenging. All E. coli can be recovered easily from clinical specimens on general or selective media. E. coli strains then can be assigned to one of the diarrheagenic pathotypes by identification of the defining phenotypes, but this identification is accomplished more commonly by DNA hybridization or by use of polymerase chain reaction (PCR) to detect specific virulence genes. In some cases, identification of a characteristic serotype can be highly suggestive of a virulent strain (e.g., O157:H7). Identification of pathotypes other than O157:H7 generally is performed in a reference laboratory.
ENTEROTOXIGENIC ESCHERICHIA COLI
Enterotoxigenic E. coli (ETEC) causes watery diarrhea, which can range from mild, self-limited disease to severe purging. The organism is a major cause of weanling diarrhea in the developing world and is the major cause of diarrhea in travelers to developing countries.
Epidemiology
ETEC is an extremely common pathogen throughout the developing world and is a ubiquitous contaminant of food and water sources in some places. Fortunately, short-lived immunity develops to ETEC surface antigens, thereby confining most symptomatic disease to immunologically naive travelers and weaning infants; in developing countries, ETEC may cause as many as one-third of cases of sporadic infant diarrhea. In endemic areas, asymptomatic infection occurs frequently. ETEC infection occurs occasionally in the United States, and several large foodborne and waterborne outbreaks have been reported.
Pathogenesis
ETEC colonizes the surface of the small bowel mucosa and elaborates enterotoxins, giving rise to a net secretory state. The enterotoxins belong to one of two groups: so-called heat-labile enterotoxins (LTs) and heat-stable enterotoxins (STs). Strains may express an LT only, an ST only, or both enterotoxins.
LTs are a class of enterotoxins that are related closely in structure and function to the cholera enterotoxin expressed by Vibrio cholerae. The LT found predominantly in human isolates (called LT-I) is approximately 75% identical at the amino acid level with cholera enterotoxin and shares several phenotypes, including its primary receptor and mechanism of action. LT-I is a toxin of approximately 86 kd and is composed of one 28-kd A subunit and five identical 11.5-kd B subunits. The B subunits are arranged in a ring and bind strongly to cell surface gangliosides GM1 and GD1b. The A subunit is responsible for the enzymatic activity of the toxin, which involves transfer of an adenosine diphosphate-ribosyl moiety from nicotinamide adenine dinucleotide to the alpha subunit of Gs, a regulatory protein complex of the basolateral membrane that serves to regulate adenylate cyclase. Adenosine diphosphate ribosylation of Gs-alpha results in adenylate cyclase being locked on, thereby leading to increased levels of intracellular cyclic adenosine monophosphate (cAMP), which, in turn, stimulate the cAMP-dependent protein kinase (A kinase) to phosphorylate and thereby activate the major chloride channel of epithelial cells, cystic fibrosis transmembrane conductance regulator (CFTR). The net result of CFTR phosphorylation is increased Cl– secretion from secretory crypt cells. Moreover, LT has been shown to inhibit NaCl absorption by villus tip cells. Increasing evidence indicates that stimulation of secretion and inhibition of absorption by LT may involve several accessory mechanisms as well.
The STs of ETEC are small, single peptide toxins that contain six disulfide bonds. Two unrelated classes of STs (STa and STb) differ in structure and mechanism of action. Only STa has been associated with human disease.
The mature STa toxin is an 18- or 19-amino-acid peptide with a molecular mass of approximately 2 kd. Two STa variants, designated STp (porcine) or STh (human), exist; both can be found among human ETEC isolates and are presumed to be equally pathogenic. STh and STp are nearly identical in the 13 residues that are necessary and sufficient for enterotoxic activity, and of these 13 residues, six are cysteines that form three intramolecular disulfide bridges.
The major receptor for STa is membrane-spanning guanylate cyclase C (GC-C), which belongs to a receptor cyclase
family that includes the atrial natriuretic peptide receptors GC-A and GC-B. Binding of STa to GC-C stimulates guanylate cyclase activity, leading to increased intracellular cyclic guanosine monophosphate (GMP), which, in turn, activates cGMP-dependent, cAMP-dependent, or both types of kinases.
family that includes the atrial natriuretic peptide receptors GC-A and GC-B. Binding of STa to GC-C stimulates guanylate cyclase activity, leading to increased intracellular cyclic guanosine monophosphate (GMP), which, in turn, activates cGMP-dependent, cAMP-dependent, or both types of kinases.
ETEC adheres to the intestinal mucosa via one or more proteinaceous fimbrial colonization factors, or CFAs. These organelles may have the appearance of rigid rods (of approximately 7 nm in diameter), thinner wiry structures, or wavy bundles of filaments. A large number of antigenically diverse CFAs have been characterized, yet epidemiologic studies suggest that approximately 75% of human ETEC worldwide express CFA-I, CFA-II, or CFA-IV.
Clinical Manifestations
The incubation period of ETEC diarrhea is 1 to 3 days in adult volunteers. Diarrhea usually begins abruptly and is watery in nature without blood, mucus, or fecal leukocytes. Patients may experience vomiting, but they generally do not have fever. ETEC infection usually is self-limited to less than 5 days.
Diagnosis
ETEC is best diagnosed by detection of the enterotoxins ST or LT. Several phenotypic and immunologic tests exist to identify the toxins. Enzyme immunoassays are available commercially. Genetic detection techniques are available in research and reference laboratories and include DNA probes and PCR.
Treatment
Maintaining adequate hydration is the cornerstone of management of ETEC diarrhea. Administration of antibiotics to which ETEC is susceptible will hasten resolution of symptoms. Trimethoprim-sulfamethoxazole has been recommended traditionally; however, increasing resistance to this agent has been documented, and alternative agents such as fluoroquinolones, ampicillin, and cefixime may be considered.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree