Complications of Total Shoulder Arthroplasty
George F. Hatch III
John G. Costouros
Peter J. Millett
Jon J.P. Warner
INTRODUCTION
Shoulder arthroplasty is a widely accepted procedure for the treatment of arthritis, fracture, nonunion, malunion, tumor, and rotator cuff arthropathy. More than 20,000 shoulder arthroplasty surgeries were performed annually in the United States in 2000 and 2001 (1). Since 1998, the total number of shoulder arthroplasties has increased by 43%. In 2003, the total number increased to approximately 25,000 shoulder arthroplasties (16,840 hemiarthroplasties and 8,150 total arthroplasties) in the United States (2). Despite these numbers, surgeons doing only one to two shoulder replacements a year perform approximately 85% of the procedures (1,2). A recent study by Jain et al. (3) reported that complications and poor outcomes of both total and hemishoulder arthroplasty were inversely proportional to the shoulder arthroplasty volume of the surgeon and the institution. As expected, surgeons and centers that had higher volumes had lower complication rates. Therefore, it is conceivable that the number of complications and the need for possible revision surgery may increase as the number of shoulder arthroplasties increases.
Accurately defining the complication rate for either total or hemishoulder arthroplasty is very difficult due to the heterogeneous populations of patients and the differences in implants used, methods of fixation, and reporting. Several authors have pooled together multiple series and have estimated complication rates for total shoulder arthroplasty (TSA) ranging from 0% to 62%. The overall mean complication rate appears to be somewhere between 8% to 16% for total shoulder arthroplasty (4, 5, 6). Godeneche et al. (4) recently reported on a homogenous series of 268 anatomically designed shoulder arthroplasties implanted for primary arthritis only, with a mean follow-up of 30 months. The authors reported an overall complication rate of 8.6%, with a 4.9% of all shoulders needing revision surgery (4).
Although the exact incidence of complications can vary depending on the initial diagnosis, several types of complications can occur with any shoulder arthroplasty. This chapter will review the most commonly seen complications with shoulder arthroplasty such as prosthetic loosening, glenohumeral instability, rotator cuff tears, periprosthetic fracture, infection, implant failure or dissociation, deltoid dysfunction, and neurovascular injury. The chapter will also provide the authors’ opinions regarding decision-making in the face of complications and approach to revision surgery.
PROSTHETIC LOOSENING
Component loosening is caused by multiple factors, often in combination. Although the shoulder joint is a typically nonweight-bearing joint, high loads can still be placed
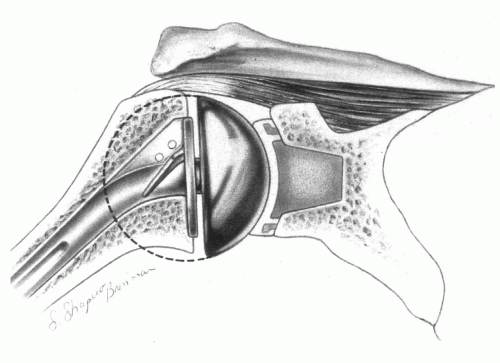
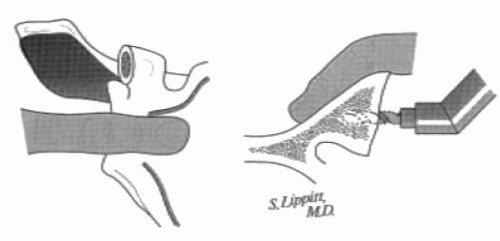
across it. For example, with the arm abducted to 90 degrees during active elevation, the load transmitted across the glenohumeral joint is approximately 90% of body weight (1). An ideal prosthetic reconstruction should minimize the risks of component wear and loosening by anatomically reconstructing the native glenohumeral geometry (Fig. 4-1). If the shoulder joint’s original center of rotation is restored, then normal kinematics and joint reactive forces are permitted, forces across the implants are minimized, and the soft tissues can perform optimally. Factors that adversely affect joint mechanics, such as component malpositioning, will alter joint function and contact stresses across the articular surface and predispose the implant to loosening.
across it. For example, with the arm abducted to 90 degrees during active elevation, the load transmitted across the glenohumeral joint is approximately 90% of body weight (1). An ideal prosthetic reconstruction should minimize the risks of component wear and loosening by anatomically reconstructing the native glenohumeral geometry (Fig. 4-1). If the shoulder joint’s original center of rotation is restored, then normal kinematics and joint reactive forces are permitted, forces across the implants are minimized, and the soft tissues can perform optimally. Factors that adversely affect joint mechanics, such as component malpositioning, will alter joint function and contact stresses across the articular surface and predispose the implant to loosening.
GLENOID COMPONENT LOOSENING
Glenoid component loosening, which is a common indication for revision surgery, occurs in approximately 2% of patients with total shoulder arthroplasty (7, 8, 9, 10, 11, 12, 13, 14, 15, 16). Glenoid loosening is a multifactor process that can be associated with component malposition, rotator cuff tears, stiffness, infection, and instability (7,17, 18, 19).
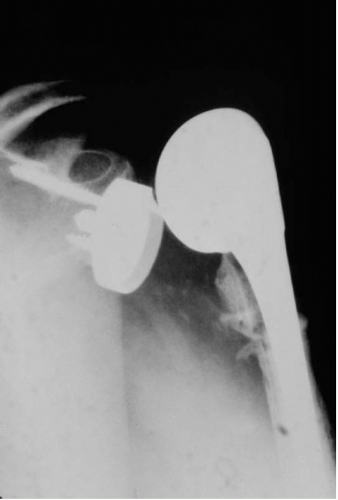
One of the main concerns with regard to glenoid loosening is the frequency of radiolucent lines around the glenoid component at follow-up (Fig. 4-2). Analysis of the literature shows a radiolucency rate ranging from 30% to greater than 90% (4,20, 21, 22, 23). In a review of 20 reported series of total shoulder arthroplasty, 38.6% of all shoulder arthroplasties had glenoid lucent lines at an average follow-up of 5 years (24). Among those shoulders with periprosthetic lucency, revision surgery was performed in only 7.7%.
Although radiolucent lines around the glenoid are common (30% to 96%), there is currently no direct evidence linking immediate radiolucent lines and clinical loosening (25,26). Reports from the Mayo Clinic have found the probability of implant survival to be 93% at 10 years and 87% at 15 years (27). At final follow-up, 79% of glenoid components developed bone-cement radiolucencies and 44% were considered to have radiographic evidence of definite glenoid loosening. The authors found a statistically significant association between loosening and increase in pain (p = 0.0001) (27). However, although the radiographic frequency of glenoid loosening was high, clinical need for revision remained low.
Glenoid loosening that is symptomatic enough to require revision surgery is uncommon, but technically challenging. Therefore, all attempts to reduce the incidence of component loosening should be made. The surgeon has little control over the bone quality of the individual. Due to the limited amount of bone in the glenoid, every attempt
should be made to preserve as much bone as possible for implant insertion. Preparation of the glenoid requires precision and meticulous attention to detail to preserve the subchondral bone while removing only enough bone to allow proper implant insertion and seating. Concentric glenoid reaming is also necessary to reduce displacement and deformation of the glenoid component (Fig. 4-3) (28). Further preparation includes pulsatile water lavage, thorough drying of glenoid bone, with or without the use of thrombin soaked gelfoam, using a vacuum system or centrifugation while mixing the bone cement to diminish porosity, pressurization of bone cement, impaction of the component into position, and maintaining stability of the component against the bone until the cement completely hardens.
should be made to preserve as much bone as possible for implant insertion. Preparation of the glenoid requires precision and meticulous attention to detail to preserve the subchondral bone while removing only enough bone to allow proper implant insertion and seating. Concentric glenoid reaming is also necessary to reduce displacement and deformation of the glenoid component (Fig. 4-3) (28). Further preparation includes pulsatile water lavage, thorough drying of glenoid bone, with or without the use of thrombin soaked gelfoam, using a vacuum system or centrifugation while mixing the bone cement to diminish porosity, pressurization of bone cement, impaction of the component into position, and maintaining stability of the component against the bone until the cement completely hardens.
HUMERAL COMPONENT LOOSENING
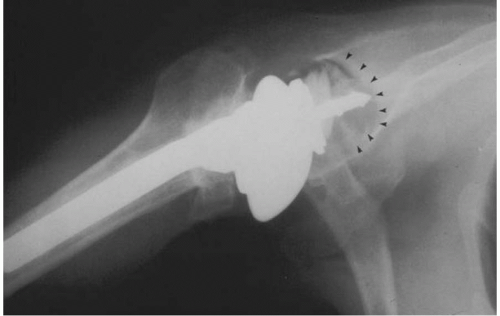
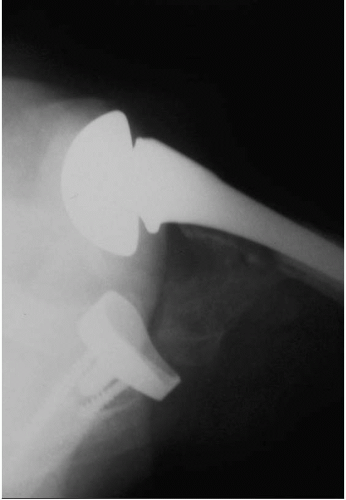
Although the majority of complications related to aseptic loosening in total shoulder arthroplasty appear to be attributed to difficulties with glenoid fixation, in short- and mid-term follow-up studies radiographic evaluations reveal a 5% incidence of implant subsidence or complete radiolucent lines measuring ≥2 mm about the humeral prosthesis (Fig. 4-4) (6,26,29, 30, 31, 32). Uncemented humeral components, as opposed to cemented humeral components, are more frequently associated with complete radiolucent lines, but clinical loosening accounted for symptoms in fewer than 2% of patients (21,22,26,29, 30, 31,33).
Cofield and colleagues (5,27) reported a 17% incidence of radiolucent line lines (≥1.5 mm) surrounding press-fit humeral head components placed for osteoarthritis. In patients with rheumatoid arthritis, 9% of the press-fit humeral components demonstrated lucent lines of ≥1.5 mm surrounding the humeral stem, with 28% of components demonstrating subsidence down the humeral canal.
Interestingly, the Mayo study (27) demonstrated no clear association between the development of symptoms and the radiographic appearance of the humeral component. The authors point out that the frequency of radiographic changes surrounding the press-fit, smooth Neer components was quite high and recommended that fixation with bone-cement be used with this type of implant.
It is apparent that press-fitting the typical designs of humeral components that do not have surface texturing or surface in-growth capabilities is not advisable. These components are generally designed for use with bone cement and therefore this is the preferred method of fixation. Although loosening of a cemented humeral component is exceedingly rare, cemented humeral components can be difficult to remove should revision surgery be necessary. Therefore, in an attempt to address this issue, new generation press-fit designs have texturing or in-growth capabilities.
IMPLANT WEAR
The correlation between osteolysis and polyethylene debris, with macrophage-induced destruction of periprosthetic
bone, has been well documented in arthoplasty literature (34). Wirth et al. (35) have described both the size and shapes of polyethylene wear debris, associated with osteolysis and recovered from failed total shoulder arthroplasty. When compared with particulate wear debris specimens from total hip revisions, polyethylene debris from total shoulder arthroplasty specimens are less round and more fibrillar (35).
bone, has been well documented in arthoplasty literature (34). Wirth et al. (35) have described both the size and shapes of polyethylene wear debris, associated with osteolysis and recovered from failed total shoulder arthroplasty. When compared with particulate wear debris specimens from total hip revisions, polyethylene debris from total shoulder arthroplasty specimens are less round and more fibrillar (35).
Recently, Gunther and colleagues (36) described a new classification system for wear analysis of glenoid components. The authors describe evidence of both surface wear and fatigue failure in retrieved glenoid components. Surface wear was noted on low-power magnification as scratching, burnishing, and abrasion, whereas fatigue failure produced component pitting, delamination, and fracture. This study demonstrates how shoulder implants can develop both surface wear and fatigue failure. These findings differ from hip and knee implants, where the modes of failure are typically wear and fatigue failure, respectively (1).
AUTHORS’ SURGICAL TECHNIQUE FOR COMPONENT LOOSENING
Removal of well-fixed components can be challenging and technically difficult. The need for careful preoperative planning in revision TSA surgery cannot be overstressed. Prior to entering the operating room, all attempts should be made to have complete knowledge of the previous procedure including component type/brand, fixation used, in addition to all previous incisions and complications. Bone and cement removal for humeral component revision surgery requires the immediate availability of high-speed, lowtorque motorized drills, multiple osteotomes of different sizes and shape, ultrasonic removal equipment, and intraoperative fluoroscopy.
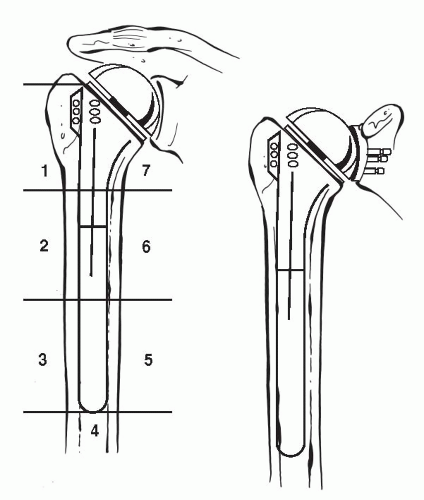
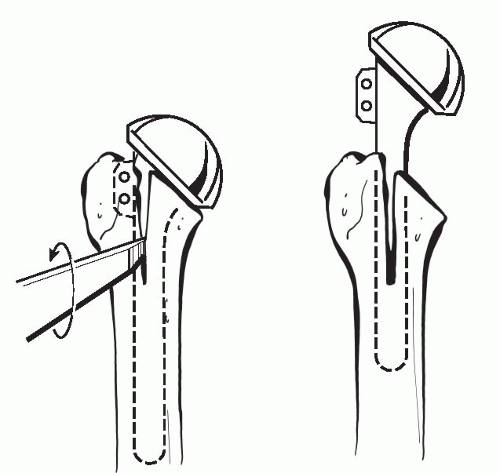
Although removal of a loose humeral stem can be quite easy with minimal damage to the humerus, extracting a well-fixed cemented or biologically in-grown prosthesis can be extremely difficult without great damage to the humeral shaft. Porous-coated stems, specifically ones where the coating extends distally, can be the most challenging of all cases. Specifically designed stem extraction devices can be of great help when removing humeral prostheses. Slap hammers and extraction devices allow the force to be applied parallel the humeral shaft, thereby decreasing the risk of humeral fracture. If the humeral stem cannot be easily removed, then a controlled osteotomy is preferred to avoid an uncontrolled iatrogenic fracture of the humerus. This is accomplished by a longitudinal anterior osteotomy just lateral to the bicipital groove of the humeral shaft, which extends approximately three-quarters of the length of the stem (Fig. 4-5) (37). Care is taken to preserve a laterally based soft-tissue hinge, which will facilitate proper closure of the osteotomy site and healing. This technique can be used for both well-fixed cemented or porous in-growth humeral stems. By slotting only the proximal aspect of the humerus, the risk of radial nerve injury can be minimized. This is due to the fact that the majority of standard-length stems lie above the spiral groove of the humerus. After the cemented stem has been removed, we either recement within the existing mantle (nonseptic failure) or remove all remnants of the cement mantle, including the cement restrictor if possible. An ultrasonic cement removal device operated under fluoroscopic guidance can be helpful. All attempts are then made to remove loose particles by pulsatile lavage and suction, followed by meticulous hemostasis. After complete preparation of the humerus, the osteotomy is secured by using multiple cerclage sutures or wires.
Prior to injecting cement, hand reaming is used to prepare the humeral canal to avoid the risk of shaft fracture. We have had two cases of radial nerve palsies during revision surgeries involving a well-fixed humeral prosthesis. One case occurred secondary to cement extrusion, and the other resulted from humeral shaft fracture during placement of the new humeral implant. For these reasons we feel intraoperative fluoroscopy is critical for evaluation of the integrity of the humeral shaft during cement removal and new prosthesis placement. If we still have doubt regarding the integrity of the shaft after fluoroscopic evaluation, we will inject saline mixed with radio-opaque dye down the shaft and look for extrusion under fluoroscopy.
Based on the quality of the remaining bone in the proximal humerus, a long-stem prosthesis is often used in revision cases. In the case of bone deficiencies, cancellous bone-grafting is used in the proximal metaphysis, and allograft fibular struts secured with cercalge wires are used to
re-enforce the fixation distally (Fig. 4-6). During placement of the new humeral prosthesis, low-viscosity cement is inserted to avoid excessive canal pressurization.
re-enforce the fixation distally (Fig. 4-6). During placement of the new humeral prosthesis, low-viscosity cement is inserted to avoid excessive canal pressurization.
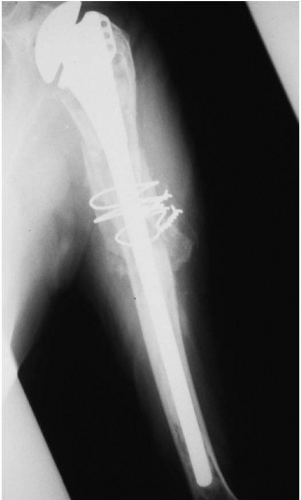 Figure 4-6 Radiograph of humeral prosthesis revision procedures with long-stem component, allograft struts, and cerclage wires. |
Access to the glenoid, in the presence of a well-fixed humeral component, can be a difficult situation. In cases where the well-fixed humeral stem is not modular, the techniques described earlier must often be used to gain adequate access to the glenoid component. However, in cases where a modular humeral prosthesis does not need to be revised, removal of the head alone allows for inspection of the glenoid. Here adequate soft-tissue release is the key to allowing optimal glenoid visualization while minimizing the risk of fracturing the humeral proximal metaphysis and/or humeral shaft.
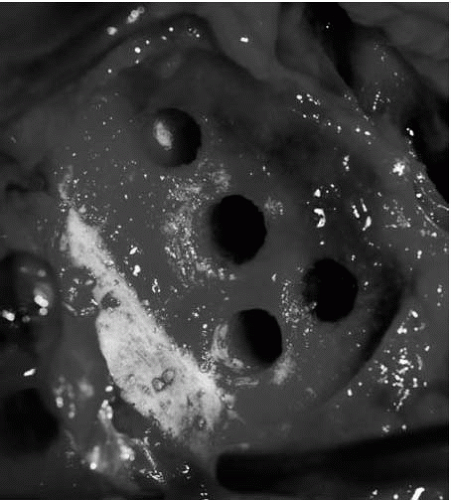
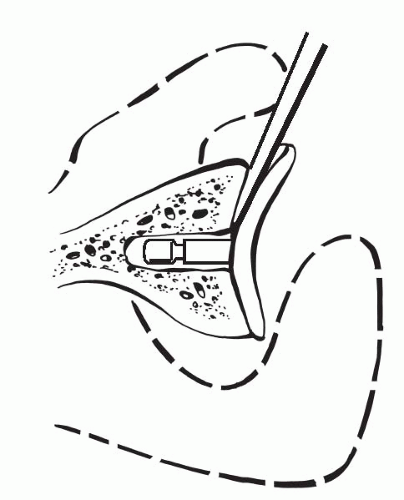
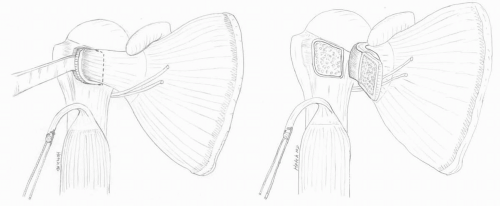
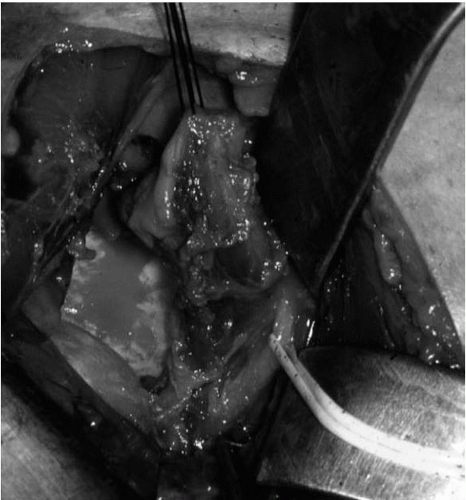
Often, a loose glenoid implant can easily be extracted from the glenoid vault without much difficulty. When a well-fixed glenoid component needs to be revised, a sharpcurved osteomes can be used to cut the articulating component away from the underlying pegs or keel (Fig. 4-7) (38). Once the articulating surface of the glenoid implant has been detached and removed, small-curved curettes, thincurved osteomes, and/or a high-speed drill are used to address the remaining pieces. Glenoid bone loss after removal of the glenoid component is not uncommon, but every effort should be made to preserve the underlying bone-stock during component and cement removal.
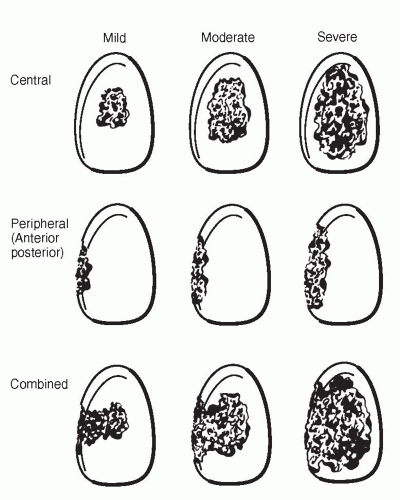
Glenoid bone loss has been categorized on the basis of location and severity (Fig. 4-8) (7). The classification system and recommendations by Antuna et al. (7) give useful guidelines for surgical decision making when dealing with glenoid bone deficiencies. In cases where there are mild to moderate central or peripheral glenoid bone deficiencies,
primary replacement of a new glenoid component, with or without bone-grafting, is often done. However, when severe central or combined deficiencies exist, implantation of a new component may not be possible. In these cases, staged grafting procedures are used by placing cancellous autograft and/or allograft into glenoid bone defects (Fig. 4-9). Therefore, a hemiarthoplasty is performed without placing a glenoid component. After graft consolidation is evident by radiographs, typically in 6 to 12 weeks, a new glenoid component can be placed. In some cases, immediate solid fixation possible primary glenoid reconstruction can be accomplished, without the need for a staged procedure (Fig. 4-10).
primary replacement of a new glenoid component, with or without bone-grafting, is often done. However, when severe central or combined deficiencies exist, implantation of a new component may not be possible. In these cases, staged grafting procedures are used by placing cancellous autograft and/or allograft into glenoid bone defects (Fig. 4-9). Therefore, a hemiarthoplasty is performed without placing a glenoid component. After graft consolidation is evident by radiographs, typically in 6 to 12 weeks, a new glenoid component can be placed. In some cases, immediate solid fixation possible primary glenoid reconstruction can be accomplished, without the need for a staged procedure (Fig. 4-10).
Extreme care is needed when preparing the glenoid vault, and every effort should be made to protect the glenoid cavity wall from penetration. Palpation with the index finger of the opposite hand on the anterior aspect of the scapular neck can give the surgeon a better idea with regard to glenoid orientation and cavity thickness (Fig. 4-11). The glenoid cancellous bed is then cleaned of debris with pulse lavage and careful drying prior to cement insertion. A syringe is used to insert the cement in a liquid state and to obtain pressurization. Cement is also directly applied to the back of the glenoid component prior to insertion. The glenoid component is then inserted and lightly impacted into the glenoid canal. Manual thumb pressure is then used to compress the implant in place until polymerization of the cement is complete.
GLENOHUMERAL COMPONENT INSTABILITY
Instability following shoulder arthroplasty is a relatively common and potentially devastating complication with reports of frequency varying from 0% to 35% (1,5,6,39, 40, 41, 42).
In a literature review of 23 shoulder arthroplasty series, instability was the most commonly reported complication with a combined rate of 5.2% (43). Instability can occur acutely in the postoperative period or present slowly after several months to years. Instability following shoulder arthroplasty can range from mild subluxation to complete and fixed dislocation (Fig. 4-12). Chronic fixed dislocations occur, as do recurrent dislocations. Like nonprosthetic instability, several authors have classified the direction of instability based on the primary direction: anterior, superior, posterior, or inferior (5,6,44,45). As in nonprosthetic instability, there is debate between authors regarding classification and etiology. For example, superior instability resulting from dynamic muscle dysfunction, isolated tears and attenuation of the supraspinatus, failed rotator cuff repair, and complete rupture of the insertion of the rotator cuff are also included into the category of glenohumeral instability by some authors (15). However, other authors feel that although superior instability can be conceptually included in the instability category, superior instability along with inferior instability should be more appropriately analyzed in the setting of massive cuff deficiency and insufficient bone-stock, respectively (46). For organizational sake, this chapter will group both superior and inferior instability in the glenohumeral instability section, realizing there can be inherent differences in the mechanisms that lead to different types of instabilities.
In a literature review of 23 shoulder arthroplasty series, instability was the most commonly reported complication with a combined rate of 5.2% (43). Instability can occur acutely in the postoperative period or present slowly after several months to years. Instability following shoulder arthroplasty can range from mild subluxation to complete and fixed dislocation (Fig. 4-12). Chronic fixed dislocations occur, as do recurrent dislocations. Like nonprosthetic instability, several authors have classified the direction of instability based on the primary direction: anterior, superior, posterior, or inferior (5,6,44,45). As in nonprosthetic instability, there is debate between authors regarding classification and etiology. For example, superior instability resulting from dynamic muscle dysfunction, isolated tears and attenuation of the supraspinatus, failed rotator cuff repair, and complete rupture of the insertion of the rotator cuff are also included into the category of glenohumeral instability by some authors (15). However, other authors feel that although superior instability can be conceptually included in the instability category, superior instability along with inferior instability should be more appropriately analyzed in the setting of massive cuff deficiency and insufficient bone-stock, respectively (46). For organizational sake, this chapter will group both superior and inferior instability in the glenohumeral instability section, realizing there can be inherent differences in the mechanisms that lead to different types of instabilities.
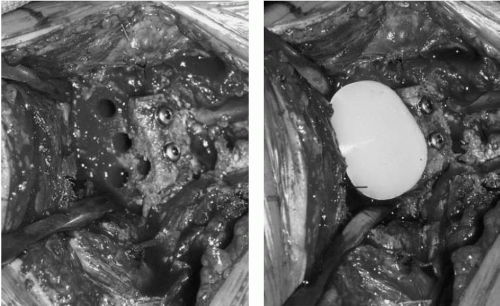 Figure 4-10 Intraoperative picture of bone grafting and fixation of glenoid defect prior to glenoid component placement. |
An extensive review of the existing literature on instability following shoulder unconstrained total shoulder arthroplasty and hemiarthroplasty have been reported by different authors (5,6,15,43,47). When broken down by direction of instability, reported rates of anterior instability
range from 18% to 43% of cases (5,6,23,33,45,48, 49, 50, 51, 52); superior instability ranges from 22% to 29% of cases (5,29,53); posterior instability ranges from 20% to 27% (5,17,26,30,33,45,54, 55, 56, 57); inferior instability ranged from 4% to 5% of cases (5,58,59); and multidirectional instability ranged from 3% to 4% (5,27,60).
range from 18% to 43% of cases (5,6,23,33,45,48, 49, 50, 51, 52); superior instability ranges from 22% to 29% of cases (5,29,53); posterior instability ranges from 20% to 27% (5,17,26,30,33,45,54, 55, 56, 57); inferior instability ranged from 4% to 5% of cases (5,58,59); and multidirectional instability ranged from 3% to 4% (5,27,60).
It is important to note that due to the relatively small number of cases of shoulder arthroplasty, the complication rates often quoted are typically from pooled series with heterogeneous populations with regard to initial patient diagnosis, indications for surgery, and implants used. It is also important to note that complication rates for constrained total shoulder prostheses should not be included when discussing results regarding complications of total shoulder arthroplasty and hemishoulder arthoplasty. Constrained total shoulder prostheses have had only limited clinical success and have been associated with more complications than unconstrained prostheses (15). Due to the overwhelming number of complications reported in the literature, many authors question the efficacy of constrained total shoulder arthroplasty, even as a salvage procedure (15).
ANTERIOR INSTABILITY
Anterior instability after shoulder arthroplasty should be considered a subscapularis tear until proven otherwise (Fig. 4-13). Subscapularis disruption after shoulder arthroplasty has been attributed to poor tissue quality, poor technique of subscapularis release and reattachment, oversized components that increase lateral humeral offset and create increased tension on the cuff, and inappropriate or overly aggressive physical therapy in the early postoperative period (5,6,15). Other causes of anterior instability include relative anteversion of the humeral component, anteversion of the glenoid component, an oversized humeral head, and anterior deltoid dehiscence (5,15).
Patients undergoing shoulder arthroplasty who develop osteoarthritis secondary to multiple procedures for anterior instability are at increased risk for complications postoperatively, when compared to patients undergoing arthroplasty without a history of instability. Matsoukis et al. (61) recently reported a complication rate of 18% for shoulder arthroplasty performed for osteoarthritis after anterior instability procedures; over half the complications were related to prosthetic instability. The type of prior instability surgery and preoperative active external rotation was not predictive of outcome. Factors correlated significantly with poor outcome were fatty degeneration of the rotator cuff muscles, particularly the subscapularis.
Matsoukis et al. (62) recently expanded on their previous study to include patients with a history of anterior instability, but without prior stabilization surgery, who underwent shoulder arthroplasty for osteoarthritis. No significant differences in demographic factors, prearthroplasty function, postarthroplasty function, prearthroplasty radiographic findings, postarthroplasty radiographic findings, complication rate, or reoperation rate were noted between
the patients treated with a prior operation for the anterior instability and those treated nonoperatively. Negative prognosticators included an older age at the time of the initial dislocation and rotator cuff tear. This investigation demonstrated that good results are obtainable with shoulder arthroplasty for the treatment of arthritis following anterior shoulder instability.
the patients treated with a prior operation for the anterior instability and those treated nonoperatively. Negative prognosticators included an older age at the time of the initial dislocation and rotator cuff tear. This investigation demonstrated that good results are obtainable with shoulder arthroplasty for the treatment of arthritis following anterior shoulder instability.
Recently, Sanchez-Sotelo and colleagues at Mayo (46) reported on 33 shoulder arthroplasties revised for anterior instability (19 shoulders) and posterior instability (14 shoulders) following shoulder arthroplasty between 1985 and 1999. The subscapularis was ruptured in 79% (15 of 19) of shoulders with anterior shoulder instability. All cases with anterior instability also had abnormal capsular compliance of some form (either too tight or too loose), two cases showed excessive glenoid component anteversion, and one case demonstrated anterior and superior instability associated with a neck cut angled too posterior and inferiorly.
To the best of our knowledge, the recent study by Sanchez-Sotelo et al. (46) has the largest number of reported anterior instability cases (19) and posterior instability cases (14) postarthoplasty from a single series to date. Of concern is the fact that more than 50% of the shoulders (23 out of 33) remained unstable despite revision surgery. Of these, 19 of 23 shoulders (83%) were considered failures. Of the 19 shoulders that were considered to be failures, 14 (74%) were cases of anterior instability. The preoperative direction of instability (anterior instability) was the only factor that was significantly associated with the final outcome of revision surgery (p = 0.04) (46). Based on these findings, the authors concluded that surgical treatment for instability, primarily in the anterior or posterior direction, following shoulder arthroplasty is associated with only limited success.
AUTHORS’ SURGICAL TECHNIQUE FOR ANTERIOR INSTABILITY
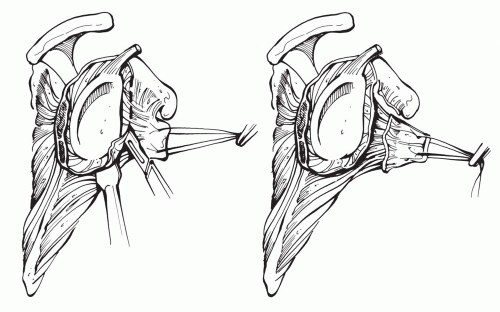
In our view, anterior instability represents a subscapularis tear until proven otherwise. In addition to addressing the subscapularis tear, the cause of the tear must also be sought and corrected if possible. The best method is primary prevention. We perform a lesser tuberosity ostetomy, which we have shown to be biomechanically superior to other methods of fixation (Fig. 4-14) (unpublished data, manuscript in preparation). Osteotomy of the lesser tuberosity permits bone-to-bone healing and decreases our risk of subscapularis disruption and dysfunction postoperatively. In addition, an anatomical head is preferred to avoid overstuffing and placing undue stress upon the rotator cuff.
When subscapularis ruptures do occur, tears are commonly found in association with oversized humeral heads, where the increased strain on the tendon can lead to attenuation and frank tearing. In addition, overly aggressive physical therapy in the early postoperative period is also has a high association with subscapularis tears (38). Therefore, both of these possible causes must be investigated. Traumatic cause such as after a fall or during heavy lifting can usually be elicited from the history.
If a tear is identified, particularly if it occurs in the early postoperative period, we advocate primary surgical repair without undue delay. Repair of a subscapularis rupture entails aggressive circumferential mobilization of the subscapularis and repair with nonabsorbable sutures (Fig. 4-15). We do not lengthen the subscapularis through Z-lengthening techniques or other methods, as this thins out and weakens the tendon. Instead we attempt to fully mobilize the remaining tendon and muscle belly by a 360-degree circumferential release of all adhesions, after isolating and protecting the axillary nerve (Fig. 4-16). Although described by Moeckel et al.
(52), we have not had success with the use of bone-tendon allograft for subscapularis reconstruction. Instead, if the subscapularis remains insufficient after complete mobilization, we opt to transfer the pectoralis major muscle to the lesser tubersosity (Fig. 4-17) (63).
(52), we have not had success with the use of bone-tendon allograft for subscapularis reconstruction. Instead, if the subscapularis remains insufficient after complete mobilization, we opt to transfer the pectoralis major muscle to the lesser tubersosity (Fig. 4-17) (63).
As stated earlier, instability following arthroplasty is typically a multifactor process. Therefore, success at revision surgery will only be achieved with a careful evaluation of the existing arthroplasty, including the soft tissue, bone stock, and components version and fixation, so that the cause of the instability can be determined.
Evaluation of component positioning and stability is performed with standard shoulder radiographs, which should always include an axillary view. A malpositioned humeral component in anteversion increases anterior humeral head translation and the possibility of anterior instability (Fig. 4-18) (64). Computed tomography (CT) scan aids in the evaluation of the unstable arthroplasty, and in our opinion is a mandatory study prior to undertaking a revision arthroplasty procedure. Because ultimately the component version is best assessed by intraoperative evaluation, final decision regarding the need to change component version is made on the operating table. Therefore all instrumentation for component revision must be available, even if only soft-tissue issues are believed to be the primary cause of instability.
SUPERIOR INSTABILITY
Superior humeral head migration is a common complication after shoulder arthroplasty. Progressive superior humeral head migration is suggestive of superior instability. Superior instability can be associated and/or caused by dynamic muscle dysfunction, attenuation of the supraspinatus muscle-tendon unit, frank rupture of the rotator cuff, failed rotator cuff repair, and coracoacromial arch insufficiency (1,5,6,15,21,26,29,53).
Boyd and associates (39) noted proximal humeral migration in 29 (22%) of 131 total shoulder arthroplasties. Rotator cuff tears were present in only 7 (24%) of the 29 cases of proximal humeral migration. Proximal humeral migration in shoulders without rotator cuff tears was attributed to an imbalance in the force couple between the strong deltoid and a weak, poorly rehabilitated rotator cuff. Boyd and associates (39) also noted that in patients with proximal humeral migration and rotator cuff tears, the amount of proximal component migration was independent of tear size. However, there was a direct correlation between proximal
humeral component migration and poor preoperative function in the setting of rotator cuff deficiency.
humeral component migration and poor preoperative function in the setting of rotator cuff deficiency.
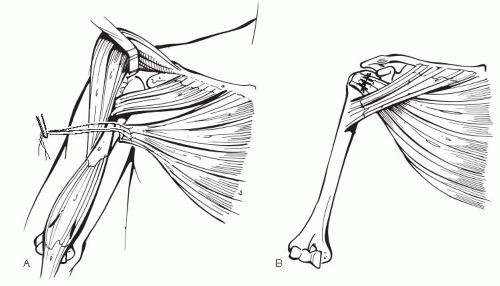 Figure 4-17 Illustration of split pectoralis major transfer. A: Harvest of sternal head. B: Transfer of sternal head to greater tuberosity. |
Cofield and associates (5) analyzed 50 shoulders which required revision surgery for instability; superior instability accounted for 17 (34%) of the cases. The causes for superior instability were supraspinatus tearing or stretching, greater tuberosity nonunion, excessive humeral component anteversion, and polyethylene dissociation (5). Unfortunately, following surgical treatment for instability in the superior direction, four patients continued to have moderate subluxation and 13 patients had severe subluxation. The results were rated as satisfactory in two patients and unsatisfactory in 15 patients.
Some authors have found that with hemiarthroplasty, progressive proximal humeral head migration was not directly related to the development of pain or prosthesis failure (39). When comparing total shoulder versus hemiarthroplasty, several authors (6,39) have noted no increased pain with proximal migration of the humeral head in patients with humeral arthoplasty alone. However, with total shoulder arthroplasty, progressive proximal migration of the humeral component causes glenoid component loosening due to eccentric loading of the component (65). Based on their observations, Barrett and associates(26) hypothesized that a superiorly migrating humeral component results in an eccentrically applied glenoid compressive force, which causes increased stress at the glenoid bone-cement interface, and therefore eventual loosening of the glenoid implant within the glenoid fossa. An association between completely irreparable rotator cuff tears, superior humeral head migration, and glenoid component loosening was also reported in seven cases of failed total shoulder arthroplasty by Franklin and colleagues (18). The authors found that the amount of proximal humeral migration positively correlated with the degree of superior glenoid component tilting and loosening. The authors coined the term the “rocking horse” (18) of the glenoid component to describe this phenomenon. Several authors have noted the association between symptomatic loosening of the glenoid component, irreparable rotator cuff tears, and glenohumeral instability (26, 56,65, 66, 67). For the reasons mentioned above, it is therefore generally agreed that, in the setting of a massive irreparable rotator cuff tear, a traditional glenoid component should not be implanted (5,6,68).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree