Complications of Fracture Management
Theodore F. Schlegel
Patrick N. Siparsky
Richard J. Hawkins
INTRODUCTION
This chapter focuses on identifying the potential complications that are encountered when caring for patients with fractures of the proximal humerus and clavicle. By becoming familiar with these potential problems, it is our hope that most of these adverse outcomes can be avoided. As has been stated so eloquently in the past, “An ounce of prevention is worth a pound of cure.” This is particularly relevant when managing difficult fractures of the shoulder girdle. For operative treatment, it is the surgeon’s obligation to discuss all the potential complications as part of an informed consent. If the unfortunate situation arises in which an undesirable outcome occurs, it is important to have an array of options to deal with these situations. It is our goal to provide strategies to avoid these situations and advice on what to do if they do happen.
COMPLICATIONS OF OPERATIVE MANAGEMENT IN SHOULDER GIRDLE FRACTURES: NONSPECIFIC TO FRACTURE TYPE
There have been many reported complications following open treatment of clavicle and displaced proximal humeral fractures. For discussion purposes, it is easiest to think of these in one of two ways: nonspecific complications that can occur regardless of the fracture pattern and location, including infection, neurovascular injuries, hardware failure, joint stiffness, and heterotopic ossification; or fracture-specific complications that are known to occur secondary to the type of fracture and location, including problems such as malunions, nonunions, or avascular necrosis. Avascular necrosis is rarely seen in two- and three-part fractures, but is more likely to occur in four-part displaced proximal humeral fractures (1).
Infection
Infection is rare with open reduction and internal fixation (ORIF) of fractures involving the shoulder girdle. Fortunately, the shoulder girdle has adequate soft-tissue coverage with an extensive vascular supply to the tissues, decreasing the risk of infection. However, infection is still possible, and it is for this reason that care should be taken to maintain sterility, administer prophylactic antibiotics, and minimize excessive soft-tissue dissection. Obtaining homeostasis at the time of closure and appropriately draining the wound (particularly in proximal humeral fractures) is important in preventing hematoma formation, which increases the risk of infection.
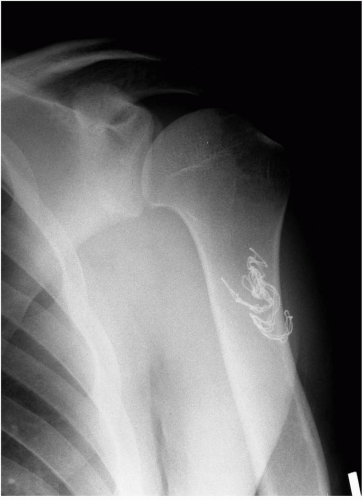
If the infection occurs early in the postoperative period, the patient may present with a constellation of findings from increased pain, decreased motion, erythema, and skin temperature changes, as well as systemic complaints of fever/chills, loss of appetite, and fatigue. If an infection is suspected, then a thorough workup is warranted. This should include a panel of blood tests including a complete blood cell count with differential, erythrocyte sedimentation rate, and a C-reactive protein measurement. Although these tests may be suggestive of an infection, the combination of results are probably the most important when attempting to make the diagnosis. Imaging should include
plain film radiographs to look for the possibility of a retained foreign body such as a surgical sponge or for any early loosening of surgically implanted hardware (Fig. 5-1). Other studies such as magnetic resonance imaging (MRI) with gadolinium can be helpful in difficult situations and may be useful in surgical planning, particularly if an abscess is identified. However, the difficulty of using an MRI comes with the imaging artifact that occurs with metal implants.
plain film radiographs to look for the possibility of a retained foreign body such as a surgical sponge or for any early loosening of surgically implanted hardware (Fig. 5-1). Other studies such as magnetic resonance imaging (MRI) with gadolinium can be helpful in difficult situations and may be useful in surgical planning, particularly if an abscess is identified. However, the difficulty of using an MRI comes with the imaging artifact that occurs with metal implants.
In cases of deep infection, it is necessary to address this problem early with a formal open irrigation and debridement. With proximal humerus fractures, arthroscopic treatment alone is usually not sufficient to treat these problems because the infection usually involves both the joint and more superficial structures. At the time of surgery, both aerobic and anaerobic cultures should be obtained. Early infection within the first 2 weeks is frequently the result of a Staphylococcus aureus infection (2). In more indolent cases with a later presentation, one should consider causes such as Peptostrepococcus. In this situation, it is often necessary to request the lab culture specifically for this organism. Positive results may take up to 7 days in certain circumstances.
Regardless of the suspected organism, the principals of a complete and thorough surgical debridement should be adhered to specifically. This includes a sharp resection of the skin edges back to healthy bleeding tissues, followed by a similar treatment of the deeper layers. Inspection of the joint should be completed, particularly in cases involving the proximal humerus where the joint has been previously violated. A pulsed lavage device using a bacteriostatic solution such a bacitracin is recommended (3). The hardware should be retained in these situations if there is no gross loosening. The wound is closed over heavy drains, usually left in place for at least 48 hours or until there is minimal output. A heavy monofilament absorbable suture is recommended for closure, minimizing the risk of harboring residual bacteria with a braided-type stitch. For long-term function, it is important to try to preserve the integrity of the rotator cuff if possible by repairing the tissue. If the tissue is of poor quality or there is excessive tension on the repair, then it is better to avoid repair.
Neurovascular Injuries
Neurovascular injuries have been well documented following both clavicle and displaced proximal humeral fractures. With clavicle fractures, neurovascular compromise often is the result of abundant callus, malunion, or fracture displacement. The vascular structures that have been reported to be involved in compression syndromes include the carotid artery and subclavian vein and/or artery. The brachial plexus can be injured as a result of a neuropraxia or progressive compression affecting the medial cord, which often produces primarily ulnar nerve symptoms (4).
Complications can also occur from hardware placement or abnormal migration, particularly with intramedullary fixation devices. Injuries have been reportedly associated with Hagie pins migration to the aorta and subclavian artery (5). Hence, care must be taken in pin placement and observation of pin location postoperatively should be noted.
With displaced proximal humeral fractures, Stableforth (6) reported a 5% incidence of axillary artery compromise and a 6.2% incidence of brachial plexus injuries. Vascular injury is most often associated with penetrating or violent blunt trauma caused by the initial injury, but also occurs after ORIF (7). If a vascular disruption occurs, the lesion is usually found at the junction of the anterior humeral circumflex artery and axillary artery. The diagnosis is often difficult to make because peripheral pulses may be normal as a result of collateral circulation. Paraesthesis may be a helpful clinical finding. Because early diagnosis and repair are crucial to the outcome, angiography and exploration should be performed immediately when a vascular injury is suspected.
The axillary nerve is susceptible to injury following fractures and surgical treatment. The axillary nerve provides motor supply to the deltoid and teres major muscles with sensory distribution over the lateral aspect of the upper arm. Sensation over the lateral deltoid region is not a reliable means of determining if there is an axillary nerve injury. A more reliable means of testing the axillary nerve is palpating all three leaves of the deltoid muscle for contraction. Due to pain in an acute fracture, this is often difficult to
accurately assess. Immediate surgical intervention is considered in cases where there is felt to be the possibility of iatrogenic injury caused by a trauma from a suture around the nerve or entrapment of the nerve under the hardware. However, most of these injuries are secondary to a neuropraxia and improve with time.
accurately assess. Immediate surgical intervention is considered in cases where there is felt to be the possibility of iatrogenic injury caused by a trauma from a suture around the nerve or entrapment of the nerve under the hardware. However, most of these injuries are secondary to a neuropraxia and improve with time.
Electromyelography should be performed if a nerve injury is suspected. This study should be obtained no earlier than 3 weeks after the injury where the results are more accurate, both for documentation and as a baseline for subsequent comparisons of recovery. If a complete axillary nerve injury does not improve within a 3-month period, surgical exploration should be considered.
Stiffness
Postoperative stiffness is not uncommon after surgical treatment of these complex fractures (Fig. 5-2). Factors contributing to the development of postsurgical stiffness include extensive soft-tissue trauma, delay in surgical intervention, necessary protection of comminuted bone fragments, and inability to aggressively institute active and passive motion until satisfactory early healing has occurred. Early passive range-of-motion exercises and careful patient selection minimize the likelihood of the development of stiffness.
If arthrofibrosis develops, surgical intervention can be considered if the patient fails to progress with an aggressive, well-supervised rehabilitation program. It is our preference to avoid operative treatment within the first 12 weeks following surgery for two reasons. First, despite instituting early patient restrictions, it is often possible to make gradual gains in motion with joint distraction techniques and terminal stretching programs. Careful documentation of motion measurements is critical in determining if progress is being made. Even if only small gains are seen, it is reasonable to continue with the program. Only after the patient plateaus is it necessary to consider surgical intervention. Second, because of the ongoing inflammatory healing cascade that is present during the first 3 months after surgery, it is better to delay an additional operation, which would create a second traumatic event and a further inflammatory process.
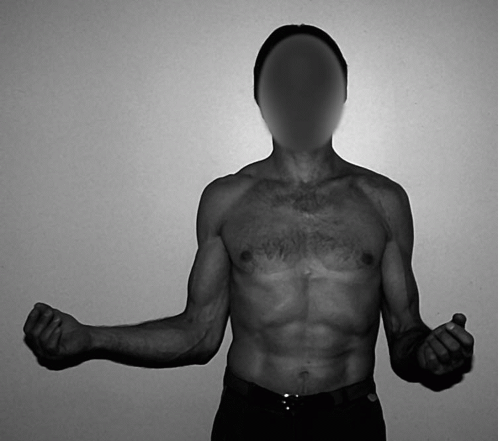 Figure 5-2 Clinical presentation of significant restriction of external rotation of the left shoulder. |
In our experience with patients who do not progress with therapy, it is critical to determine whether the loss of motion represents a functional disability. This may vary greatly between patients depending on their preinjury level of activity. Realistic goals should be set because the outcome of surgery may be unpredictable. Only after that time is it reasonable to consider surgical intervention. As opposed to idiopathic arthrofibrosis, postsurgical stiffness is rarely responsive to manipulation alone. This method of treatment is unpredictable and potentially fraught with problems.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree