Even with current techniques and instrumentation, complications can occur after operative treatment of adolescent idiopathic scoliosis. The most dreaded complications—neurologic deficits—are relatively infrequent, occurring in 1% or less of patients. Nonneurologic deficits, such as infection, pseudarthrosis, curve progression, and proximal junctional kyphosis, are more frequent, but are much less likely to require reoperation or to cause poor functional outcomes. Understanding the potential complications of surgical treatment of pediatric spinal deformity is essential for surgical decision-making.
Key points
- •
With current instrumentation and techniques, including intraoperative neuromonitoring, neurologic complications are relatively infrequent (<1%), and most new neurologic deficits resolve without treatment.
- •
Infection is perhaps the most frequent reason for unanticipated repeat surgery after primary spinal fusion for adolescent idiopathic scoliosis; whether implant removal is necessary remains controversial.
- •
The most common areas of pseudarthrosis are at the thoracolumbar junction and at the distally fused segment.
- •
Curve progression (the adding-on phenomenon) and the crankshaft phenomenon occur primarily in younger patients; their effect on clinical outcomes is unclear.
- •
Proximal junctional kyphosis is more common in adults than in adolescents with idiopathic scoliosis; it seems to have little or no effect on functional outcomes.
Adolescent idiopathic scoliosis (AIS) is the most extensively investigated pediatric deformity, with numerous studies reporting surgical complications and reoperation rates. The frequency of complications after AIS surgery is affected by many variables, and existing information in the literature on surgical complications remains limited because of small numbers of patients, focus on a single procedure or complication, outdated surgical techniques, and confinement to a single surgeon or center experience. Understanding the potential complications of surgical treatment of pediatric spinal deformity is essential for surgical decision making.
Although current instrumentation and techniques produce good long-term surgical outcomes in most patients, complications do occur. Complications can be broadly categorized as neurologic or nonneurologic, with reported frequencies ranging from 7% to 15% for nonneurologic complications and generally less than 1% for neurologic complications, which are the most devastating.
Neurologic complications
Although much less frequent than nonneurologic complications, neurologic complications (eg, nerve root, cauda equina, spinal cord deficit) are the most feared, because they can result in complete or partial paralysis, peripheral nerve deficits, or rarely death. Causes of neurologic complications include extrinsic compression of the spinal cord by implants, an epidural hematoma or abscess, or iatrogenic injury to neural elements; distraction of the spinal cord during correction; and ischemic injury that results in reduced blood supply to the spinal cord.
A 2011 report from the Scoliosis Research Society (SRS) Morbidity and Mortality Committee identified neurologic complications in 0.8% of 11,227 patients with AIS, most commonly incomplete spinal cord deficits and nerve root deficits; complete spinal cord deficits were rare and there were no cauda equina deficits. All nerve root deficits and complete spinal cord deficits had complete or partial recovery; only 1 of 41 incomplete spinal cord deficits had no recovery. A metaanalysis including 1136 patients with AIS treated with instrumented posterior spinal fusion found neurologic complications in only 2 patients (0.17%), both of whom had complete recovery.
Reported risk factors for neurologic complications in AIS surgery include vertebral osteotomies, kyphosis correction, a Cobb angle of more than 90°, revision surgery, and combined anterior and posterior fusions. According to the 2011 SRS report, use of an osteotomy (Smith-Peterson osteotomy, pedicle subtraction osteotomy, vertebral column resection) was associated with a 2% rate of new neurologic deficits, significantly higher than the rate without osteotomy (0.9%). Compared with pedicle screw-only and hook-only constructs, wire-only and anterior screw-only constructs had significantly higher rates of new neurologic deficits.
Intraoperative neuromonitoring has become a routine part of most scoliosis surgery. The use of both somatosensory evoked potentials (SEP) and transcranial electrical stimulation-motor evoked potentials (TES-MEP) has been recommended as the most effective method for monitoring during spinal surgery. Combined SEP and TES-MEP monitoring has been reported to have a sensitivity of 100% and specificity of 98% for sensory motor impairment. In a series of 3436 consecutive pediatric spinal procedures reported by Thuet and colleagues, 74 (2.2%) potential neurologic deficits were identified; 7 patients had neurologic deficits undetected by neuromonitoring. Use of combined SEP, TES-MEP, descending neurogenic-evoked potentials, and electromyography monitoring allowed accurate detection of permanent neurologic status in 99.6% of patients.
Schwartz and colleagues defined an intraoperative “alert” or clinically relevant neurophysiologic change as a persistent (over ≥3 test trials) unilateral or bilateral loss of 65% or more of the amplitude of the TES-MEP or 50% or more of the amplitude of the SEP relative to a stable baseline. When critical changes are identified in either the SEP or TES-MEP or both, blood pressure should be elevated to a mean arterial pressure of at least 90 mm Hg, hypervolemia and blood loss should be corrected, and any operative steps that preceded the changes (eg, placement of pedicle screws, osteotomty, correction maneuver) should be reversed. The use of corticosteroids remains controversial and should be determined on a case-by-case basis. If neuromonitoring patterns return to baseline, surgery can be resumed cautiously. If amplitudes do not improve after reversal of correction and implant removal, cessation of the procedure should be considered.
Because delayed-onset neurologic deficits can occur, careful neurologic monitoring of upper and lower extremity function should be continued for 48 hours after surgery. Delayed neurologic complications may be caused by progressive spinal cord ischemia secondary to traction or to the development of an epidural hematoma. Blood pressure should be monitored carefully and mean arterial blood pressure should be maintained at greater than 80 mm Hg to help maintain spinal cord perfusion; vasopressors may be required. Hemoglobin levels should be checked and corrected, and temperature should be maintained above 36.5°C (97.7°F). Unless their acquisition would substantially delay a return to surgery, computed tomography or MRI scans are helpful in delineating the cause of the deficit.
Neurologic complications
Although much less frequent than nonneurologic complications, neurologic complications (eg, nerve root, cauda equina, spinal cord deficit) are the most feared, because they can result in complete or partial paralysis, peripheral nerve deficits, or rarely death. Causes of neurologic complications include extrinsic compression of the spinal cord by implants, an epidural hematoma or abscess, or iatrogenic injury to neural elements; distraction of the spinal cord during correction; and ischemic injury that results in reduced blood supply to the spinal cord.
A 2011 report from the Scoliosis Research Society (SRS) Morbidity and Mortality Committee identified neurologic complications in 0.8% of 11,227 patients with AIS, most commonly incomplete spinal cord deficits and nerve root deficits; complete spinal cord deficits were rare and there were no cauda equina deficits. All nerve root deficits and complete spinal cord deficits had complete or partial recovery; only 1 of 41 incomplete spinal cord deficits had no recovery. A metaanalysis including 1136 patients with AIS treated with instrumented posterior spinal fusion found neurologic complications in only 2 patients (0.17%), both of whom had complete recovery.
Reported risk factors for neurologic complications in AIS surgery include vertebral osteotomies, kyphosis correction, a Cobb angle of more than 90°, revision surgery, and combined anterior and posterior fusions. According to the 2011 SRS report, use of an osteotomy (Smith-Peterson osteotomy, pedicle subtraction osteotomy, vertebral column resection) was associated with a 2% rate of new neurologic deficits, significantly higher than the rate without osteotomy (0.9%). Compared with pedicle screw-only and hook-only constructs, wire-only and anterior screw-only constructs had significantly higher rates of new neurologic deficits.
Intraoperative neuromonitoring has become a routine part of most scoliosis surgery. The use of both somatosensory evoked potentials (SEP) and transcranial electrical stimulation-motor evoked potentials (TES-MEP) has been recommended as the most effective method for monitoring during spinal surgery. Combined SEP and TES-MEP monitoring has been reported to have a sensitivity of 100% and specificity of 98% for sensory motor impairment. In a series of 3436 consecutive pediatric spinal procedures reported by Thuet and colleagues, 74 (2.2%) potential neurologic deficits were identified; 7 patients had neurologic deficits undetected by neuromonitoring. Use of combined SEP, TES-MEP, descending neurogenic-evoked potentials, and electromyography monitoring allowed accurate detection of permanent neurologic status in 99.6% of patients.
Schwartz and colleagues defined an intraoperative “alert” or clinically relevant neurophysiologic change as a persistent (over ≥3 test trials) unilateral or bilateral loss of 65% or more of the amplitude of the TES-MEP or 50% or more of the amplitude of the SEP relative to a stable baseline. When critical changes are identified in either the SEP or TES-MEP or both, blood pressure should be elevated to a mean arterial pressure of at least 90 mm Hg, hypervolemia and blood loss should be corrected, and any operative steps that preceded the changes (eg, placement of pedicle screws, osteotomty, correction maneuver) should be reversed. The use of corticosteroids remains controversial and should be determined on a case-by-case basis. If neuromonitoring patterns return to baseline, surgery can be resumed cautiously. If amplitudes do not improve after reversal of correction and implant removal, cessation of the procedure should be considered.
Because delayed-onset neurologic deficits can occur, careful neurologic monitoring of upper and lower extremity function should be continued for 48 hours after surgery. Delayed neurologic complications may be caused by progressive spinal cord ischemia secondary to traction or to the development of an epidural hematoma. Blood pressure should be monitored carefully and mean arterial blood pressure should be maintained at greater than 80 mm Hg to help maintain spinal cord perfusion; vasopressors may be required. Hemoglobin levels should be checked and corrected, and temperature should be maintained above 36.5°C (97.7°F). Unless their acquisition would substantially delay a return to surgery, computed tomography or MRI scans are helpful in delineating the cause of the deficit.
Nonneurologic complications
The reported prevalence of nonneurologic complications after correction of AIS ranges from 0% to 30%. Nonneurologic complications can be categorized as perioperative (intraoperative and postoperative complications occurring during the first week after surgery), early postoperative complications (occurring between postoperative weeks 2 and 4), or late postoperative complications (occurring after postoperative week 4). Carreon and colleagues listed the most common nonneurologic complications in their 702 patients as respiratory complications, excessive bleeding, wound infections, and wound-related complications (eg, hematoma, seroma, dehiscence).
Infection
The reported prevalence of infection after AIS surgery ranges from 0% to 10%. Although infection is much less frequent after AIS surgery than after treatment of nonidiopathic scoliosis, it is a well-recognized complication and is one of the most common reasons for unanticipated repeat surgery after primary spinal fusion for AIS. In a 2011 report from the SRS Morbidity and Mortality Committee, the rate of wound infection in 11,741 patients with AIS was 1.4%. A metaanalysis that included 721 patients with AIS reported an almost 4% infection rate, the lowest rate with pedicle screw constructs and the highest with Harrington rods. In their analysis of 277 patients with surgical correction of scoliosis, Aleissa and colleagues found the lowest rate of deep wound infections in patients with AIS (1.5%) and the highest rate in those with neuromuscular scoliosis (14%); however, Ho and colleagues, in a series of 53 patients with infections after scoliosis surgery, found that the type of scoliosis was not a significant predictor of the development of infection.
Suggested risk factors for postoperative infection, other than a diagnosis of nonidiopathic scoliosis, include prolonged duration of surgery, higher volume of instrumentation, the use of an allograft, and combined anterior–posterior procedures. The use of instrumentation has been suggested to increase the risk of infection because of the increase in operative time and surgical exposure, the bulk of the implants, an inflammatory reaction. Aleissa and colleagues reported infection rates of 5% in patients with posterior-only surgery and 17% in those with anterior–posterior procedures. The frequency of infection was 16% with allografts and 1.4% without, although this finding has been contradicted by other studies. Blood loss, the use of perioperative medications to decrease blood loss, duration of surgery, preoperative Cobb angle, and number of levels fused were not found to be significant risk factors. Both underweight and overweight have been linked to the development of postoperative infections. First-generation stainless steel implants seem to increase the risk of delayed infection compared with newer generation titanium implants.
Delayed infection (>1 year after surgery) also can occur, with Staphylococcus aureus, S epidermis, and Propionibacterium acnes the most frequently reported infecting organisms ( Fig. 1 ). These infections are believed to result from direct seeding of the surgical field during the index procedure, followed by a latent period and then activation at some later time. Di Silvestre and colleagues reported 15 (2.77%) delayed infections at an average of 70 months (range, 15–95 ) after posterior-only fusion in 540 patients with AIS. Of the 14 deep wound infections reported by Aleissa and colleagues, 6 developed early (within 3 weeks) and 8 at a later time (average, 37 weeks; range, 5–72 ). Ho and colleagues described 21 infections after treatment of AIS, 11 of which were early and 10 were late infections, and Rihn and colleagues reported that of 7 infections (3%) in 236 patients, 1 was acute (17 days after surgery) and 6 were delayed (average 34 months after surgery).
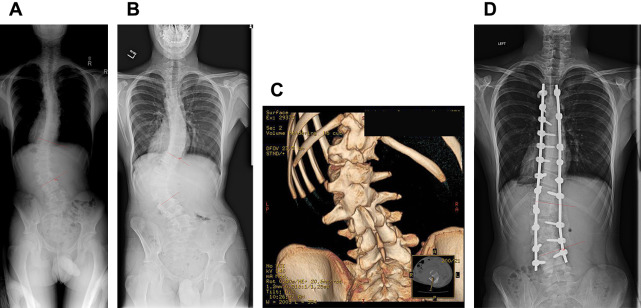
The treatment of infections after AIS surgery remains an area of controversy, with some authors recommending implant removal and others suggesting that implants should be retained to prevent progression of the deformity. Aleissa and colleagues described treatment of 8 infections with serial debridements and vacuum-assisted closure, all of which resolved without removal of implants. Two other studies support the use of vacuum-assisted closure, with none of the patients in those studies requiring implant removal. The duration of antibiotic administration necessary for infection eradication also is debatable. Earlier studies recommended a minimum of 6 weeks of intravenous antibiotics, whereas more recent studies have concluded that a much shorter course (48–72 hours of IV antibiotics, followed by 7–14 days of oral antibiotics) is just as effective.
A number of measures have been recommended for prevention of infection after AIS surgery, including preoperative administration of cefazolin, with the addition of gentamicin in high-risk groups. Myung and colleagues described 2 simple changes in their protocol that result in a 10-fold reduction in infections after posterior fusion for AIS: (1) vancomycin and ceftazidime were added to cefazolin for routine antibiotic prophylaxis, and (2) pulse lavage irrigation replaced bulb syringe irrigation intraoperatively. The use of vancomycin powder has been shown to reduce significantly the frequency of surgical site infections in adult patients ; however, Martin and colleagues found no difference in the rates of infection with and without vancomycin in 306 patients with AIS. Gans and colleagues reported 3 infections (3.4%) in 87 patients in whom vancomycin powder was used; no patient experienced nephrotoxicity or red man syndrome. A systematic review found insufficient evidence to recommend prophylaxis with intravenous vancomycin or the use of vancomycin or gentamicin powder in the surgical site or graft. An expert panel of pediatric spine surgeons and infectious disease specialists developed a Best Practice Guideline with 14 recommendations for preventing infection after pediatric spine surgery, although Glotzbecker and colleagues found insufficient evidence to recommend most of these. Aleissa and colleagues also suggested limiting the use of allograft where possible and irrigation with a betadine solution before application of the bone graft.
The clinical ramifications of postoperative infection are unclear. Several reports have noted no loss of correction or progression of deformity after implant removal. Rihn and colleagues found no differences in pain, function, self-image, satisfaction, or total SRS 24 scores between patients who developed an infection and those who did not.
Pseudarthrosis
Improvements in surgical techniques and instrumentation, the inclusion of intraarticular fusion, and meticulous dissection around the transverse processes have decreased the pseudarthrosis rate to approximately 1% in patients with AIS. A systematic review of the literature in identified a 22.7% frequency of symptomatic pseudarthrosis, most commonly after noninstrumented fusion, and a 2% to 7% frequency in instrumented fusions. A metaanalysis of 1565 instrumented posterior spinal fusions, found pseudarthroses in 2%, more frequent with Harrington rods (3%) than with Cotrel-Dubosset constructs (2%); no pseudarthroses occurred in 254 patients with all-pedicle screw fixation. The most common areas of pseudarthrosis are at the thoracolumbar junction and at the distally fused segment. With more rigid and stronger implants, pseudarthrosis may not be apparent for years. Pseudarthrosis may be identified by oblique radiographs, computed tomography, or bone scan ( Fig. 2 ); however, pseudarthrosis can be confirmed definitively only by surgical exploration. If a pseudarthrosis does not cause pain or loss of correction, surgery may not be necessary. If surgery is indicated, the pseudarthrosis is treated as any other joint to be fused: the edges are freshened and decorticated, autogenous bone graft is inserted, and instrumentation is applied.





