Abstract
Objective
Evaluation of the clinical effectiveness and safety of a new custom-made valgus knee brace (OdrA) in medial knee osteoarthritis (OA) in terms of pain and secondary symptoms.
Methods
Open-label prospective study of patients with symptomatic medial knee OA with clinical evaluation at 6 and 52 weeks (W6, W52). We systematically assessed pain on a visual analog scale (VAS), Knee injury and Osteoarthritis Outcome Score (KOOS), spatio-temporal gait variables, use of nonsteroidal anti-inflammatory drugs (NSAIDs) and analgesic-sparing effects of the brace and tolerance. Mean scores were compared at baseline, W6 and W52 and the effect size (ES) and 95% confidence intervals (95% CIs) were calculated.
Results
We included 20 patients with knee OA (mean age 64.2 ± 10.2 years, mean body mass index 27.2 ± 5.4 kg/m 2 ). VAS pain and KOOS were improved at W6 and W52: pain (ES = 0.9 at 1 year), amelioration of other symptoms (ES = 0.4), and function in activities of daily living (ES = 1.1), sports and leisure (ES = 1.5), quality of life (ES = 0.9) and gait speed (ES = 0.41). In total, 76% of patients showed clinical improvement at 1 year. Analgesic and NSAIDs consumption was significantly decreased at W6 and W52. One serious adverse effect noted was lower-limb varices, and observance was deemed satisfactory at 1 year.
Conclusion
This new unloader brace appeared to have good effect on medial knee OA, with an acceptable safety profile and good patient compliance.
1
Introduction
Knee osteoarthritis (OA) is a chronic disabling joint disease that causes increasingly severe functional impairment in everyday activities. The medial compartment is the most frequently affected, given the physiological high loading on this zone. The condition is frequently aggravated by constitutional or acquired bow-leggedness . To limit pain in medial-compartment knee OA, conservative medical management combining pharmacological and nonpharmacological treatment is recommended . The use of medical devices such as foot pronation orthotics or articulated valgus knee braces is advocated . Although the beneficial effect of these devices on symptoms are related to their proprioceptive properties or muscle activation , the principal effect stems from their ability to unload the medial compartment, where the pain originates .
The improvement in functional capacities is better with unloader knee braces than knee sleeves or neutral articulated braces . However, the efficacy of the braces is still debated , and tolerance to the braces is poor because they irritate the skin, impair venous return, can cause oedema and are bulky, which can hamper certain movements in everyday life . In clinical practice, this type of orthotic device is rarely prescribed by physicians specialized in degenerative joint diseases of the knee because they prefer pharmacological treatments and/or rehabilitation .
Recently, the PROTEOR group developed a new custom-made brace, the OdrA system ( Fig. 1 ). The brace features an innovative system to unload the medial compartment by distraction and external rotation. This mechanism allows for shifting the vertical axis of the ground reaction force vector backwards and medially toward the center of the knee joint, which reduces the knee adduction moment during the propulsion phase but disappears in the swing phase or at rest, with the knee bent. The new system, which was recently validated biomechanically in terms of kinetic and kinematic dimensions , is also less cumbersome because it is custom-made, with few voluminous tibial and femoral straps. This dynamic unloader brace, with no effect at rest with the knee bent, is equipped with a rack and pinion system that plays a dual role in weight-bearing positions: distraction and external rotation of the leg. The effect is to shift the centre of the load toward the natural inter-condyle position and thus to limit overloading of the medial compartment , which is often aggravated in patients with bow-leggedness or with medial meniscus degeneration.
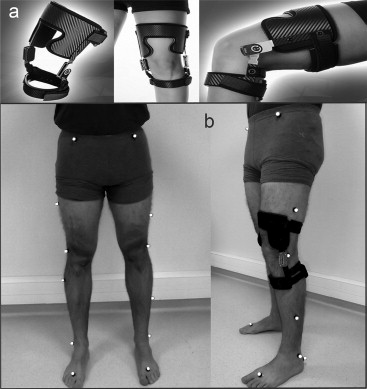
In terms of the current overall re-evaluation of treatments in knee OA, the beneficial effects of this device on symptoms by unloading the medial compartment as well as tolerance and compliance could lead to its use in clinical practice. However, in addition to data needed from validated algo-functional questionnaires, spatio-temporal gait data are needed to provide an objective evaluation of the functional benefits of this dynamic knee brace on gait . These investigations are in response to recent requests from accreditation organisations responsible for authorising the commercialisation of these medical devices: the French health authority requires a high level of scientific evidence for these orthotic devices, with high-quality therapeutic trials, on which marketing approval for these expensive and not risk-free devices depends .
The primary objective of this interventional prospective single-centre study was to evaluate the efficacy of the new valgus knee brace with the OdrA system for medial-compartment knee OA on pain at week 6 (W6). Secondary objectives were to evaluate the effect of the brace on other symptoms in the short-term (W6) and medium-term (W52) and to provide data on tolerance and compliance in clinical practice.
2
Materials and methods
2.1
Patients
Patients consulting at the Department of Rheumatology and Physical Medicine of Dijon University Hospital over six months were recruited consecutively. We included patients 40 to 80 years old who had unilateral medial-compartment knee OA according to ACR criteria (medial compartment pain at rest > 4 on a 0–10 visual analog scale [VAS]), radiological stage II, III or IV according to the Kellgren and Lawrence classification determined by radiography performed in the previous six months, with no change in pharmacological treatment in the previous six months and no injections of hyaluronic acid or corticosteroids during this period. Exclusion criteria were presence of a disease that could interfere with gait analysis or inflammatory or rapidly destructive knee OA. Patients with an indication for surgery according to the medical specialist consulted, a valgus morphotype or another disease likely to cause knee pain or modify gait were also excluded. After inclusion and custom-moulding of the OdrA brace, patients were instructed to wear the brace for at least 6 h/day, 5 days/week.
The study was conducted in accordance with good clinical practices and the Declaration of Helsinki (ClinicalTrials.gov identifier: NCT01884883 ) and was approved by the local ethics committee. Patients gave informed consent to be in the trial.
2.2
Gait protocol
At inclusion and at W6 after wearing the brace, patients underwent a standard protocol for quantified gait analysis (VICON system, Oxford, UK). This gait protocol has been described elsewhere for the biomechanical validation of the OdrA device . Briefly, reflective markers, detected by eight infrared cameras, were placed on the pelvis and lower limbs of patients, who were instructed to walk up and down a 10-m path 12 times. The spatio-temporal gait variables were recorded over the 6 m in the middle of the track to avoid acceleration and deceleration phenomena. The patients were told to walk at their usual comfortable speed.
2.3
Data collection
At inclusion, the following clinical data were collected: age, sex, body mass index (kg/m 2 ), disease duration, and radiological stage by the Kellgren and Lawrence classification .
Judgement criteria were collected at inclusion and at 6 and 52 weeks (W6, W52). For the principal outcome criteria (improvement in pain at W6 compared with inclusion), pain was measured at rest by a VAS (0–100).
The following secondary outcomes were evaluated. Improvement in pain at W52 compared with at inclusion was measured at rest by a VAS (0–100). Overall self-evaluation of disease severity was measured by a VAS (0–100). Function was measured by the Knee injury and Osteoarthritis Outcome Score (KOOS) consisting of 42 questions covering 5 domains, each scored from 0 (worst) to 100 (best) : pain, other symptoms, function in activities of daily living (ADL), function in sports and leisure (SL) activities and quality of life (QoL). This internationally validated score includes all of the domains of Western Ontario and McMaster Universities Arthritis Index (WOMAC; pain, stiffness, function) and adds more demanding activities and important aspects of QoL. The KOOS can be represented in the form of a graph, with a line linking the different domains . Consumption of nonsteroidal anti-inflammatory drugs (NSAIDs) and analgesics was evaluated by the number of days per week each class of drug was taken. Disease severity at W6 and W52 was measured by a semi-quantitative Likert scale: 1, severely worsened; 2, worsened; 3, stable; 4, improved; 5, much improved. Tolerance to the brace and compliance was evaluated by recording adverse effects in a patient diary and by mean time the brace was worn (number of hours per day and number of days per week). The following spatio-temporal gait variables were collected at W0 and W6 : walking speed (m/s), stride length (m), stride width (m), stride frequency (Hz), single and double support time (% of gait cycle) and step dephasing (% of gait cycle).
2.4
Statistical analysis
The principal analysis was intent-to-treat (ITT), with last observation carried forward (LOCF) used for missing data. Data are described with mean ± SD for clinical and gait spatio-temporal variables. Scores at different times were compared with those at inclusion by Wilcoxon matched pairs test. P < 0.05 was considered statistically significant. The amplitude of the therapeutic effect of the brace for each judgement criterion was evaluated by the effect size (ES) with the following interpretation: 0 to 0.5, weak effect; 0.5 to 0.8, moderate effect; > 0.8, major effect . For ES values (clinical and spatio-temporal parameters), 95% confidence intervals (95% CIs) were calculated by the non-parametric bootstrap method.
According to data in the literature from similar clinical studies, improvement in pain on a VAS at W6 (principal criterion) should be at least 20%. With an alpha risk of 0.05 and power of 80%, a minimum of 15 subjects was necessary. Taking into account the possibility of patients leaving the trial, we needed to include 20 patients for 1 year of follow-up. Statistical analysis involved use of Statistica v10.2 (Statsoft Inc., Tulsa, USA).
2
Materials and methods
2.1
Patients
Patients consulting at the Department of Rheumatology and Physical Medicine of Dijon University Hospital over six months were recruited consecutively. We included patients 40 to 80 years old who had unilateral medial-compartment knee OA according to ACR criteria (medial compartment pain at rest > 4 on a 0–10 visual analog scale [VAS]), radiological stage II, III or IV according to the Kellgren and Lawrence classification determined by radiography performed in the previous six months, with no change in pharmacological treatment in the previous six months and no injections of hyaluronic acid or corticosteroids during this period. Exclusion criteria were presence of a disease that could interfere with gait analysis or inflammatory or rapidly destructive knee OA. Patients with an indication for surgery according to the medical specialist consulted, a valgus morphotype or another disease likely to cause knee pain or modify gait were also excluded. After inclusion and custom-moulding of the OdrA brace, patients were instructed to wear the brace for at least 6 h/day, 5 days/week.
The study was conducted in accordance with good clinical practices and the Declaration of Helsinki (ClinicalTrials.gov identifier: NCT01884883 ) and was approved by the local ethics committee. Patients gave informed consent to be in the trial.
2.2
Gait protocol
At inclusion and at W6 after wearing the brace, patients underwent a standard protocol for quantified gait analysis (VICON system, Oxford, UK). This gait protocol has been described elsewhere for the biomechanical validation of the OdrA device . Briefly, reflective markers, detected by eight infrared cameras, were placed on the pelvis and lower limbs of patients, who were instructed to walk up and down a 10-m path 12 times. The spatio-temporal gait variables were recorded over the 6 m in the middle of the track to avoid acceleration and deceleration phenomena. The patients were told to walk at their usual comfortable speed.
2.3
Data collection
At inclusion, the following clinical data were collected: age, sex, body mass index (kg/m 2 ), disease duration, and radiological stage by the Kellgren and Lawrence classification .
Judgement criteria were collected at inclusion and at 6 and 52 weeks (W6, W52). For the principal outcome criteria (improvement in pain at W6 compared with inclusion), pain was measured at rest by a VAS (0–100).
The following secondary outcomes were evaluated. Improvement in pain at W52 compared with at inclusion was measured at rest by a VAS (0–100). Overall self-evaluation of disease severity was measured by a VAS (0–100). Function was measured by the Knee injury and Osteoarthritis Outcome Score (KOOS) consisting of 42 questions covering 5 domains, each scored from 0 (worst) to 100 (best) : pain, other symptoms, function in activities of daily living (ADL), function in sports and leisure (SL) activities and quality of life (QoL). This internationally validated score includes all of the domains of Western Ontario and McMaster Universities Arthritis Index (WOMAC; pain, stiffness, function) and adds more demanding activities and important aspects of QoL. The KOOS can be represented in the form of a graph, with a line linking the different domains . Consumption of nonsteroidal anti-inflammatory drugs (NSAIDs) and analgesics was evaluated by the number of days per week each class of drug was taken. Disease severity at W6 and W52 was measured by a semi-quantitative Likert scale: 1, severely worsened; 2, worsened; 3, stable; 4, improved; 5, much improved. Tolerance to the brace and compliance was evaluated by recording adverse effects in a patient diary and by mean time the brace was worn (number of hours per day and number of days per week). The following spatio-temporal gait variables were collected at W0 and W6 : walking speed (m/s), stride length (m), stride width (m), stride frequency (Hz), single and double support time (% of gait cycle) and step dephasing (% of gait cycle).
2.4
Statistical analysis
The principal analysis was intent-to-treat (ITT), with last observation carried forward (LOCF) used for missing data. Data are described with mean ± SD for clinical and gait spatio-temporal variables. Scores at different times were compared with those at inclusion by Wilcoxon matched pairs test. P < 0.05 was considered statistically significant. The amplitude of the therapeutic effect of the brace for each judgement criterion was evaluated by the effect size (ES) with the following interpretation: 0 to 0.5, weak effect; 0.5 to 0.8, moderate effect; > 0.8, major effect . For ES values (clinical and spatio-temporal parameters), 95% confidence intervals (95% CIs) were calculated by the non-parametric bootstrap method.
According to data in the literature from similar clinical studies, improvement in pain on a VAS at W6 (principal criterion) should be at least 20%. With an alpha risk of 0.05 and power of 80%, a minimum of 15 subjects was necessary. Taking into account the possibility of patients leaving the trial, we needed to include 20 patients for 1 year of follow-up. Statistical analysis involved use of Statistica v10.2 (Statsoft Inc., Tulsa, USA).
3
Results
We included 20 patients in the study (16 females; mean age 64.2 ± 10.2 years; mean body mass index 27.2 ± 5.4 kg/m 2 ) ( Table 1 ). Pain, disease severity and functional disability at inclusion were high, with no indication for surgery according to the treating rheumatologist. In total, 16 patients (80%) were taking level I or II analgesics and 6 (30%) NSAIDs. At W6, clinical and gait analysis data were analyzed for 19 patients because one patient had to stop wearing the brace due to venous intolerance and at W52, 18 of the 19 patients were re-evaluated (one patient lost to follow-up).
| Characteristics | |
|---|---|
| Age (years) | 64.2 ± 10.2 |
| Sex ratio (F/M), no. of patients | 16/4 |
| BMI (kg/m 2 ) | 27.2 ± 5.4 |
| Disease duration (years) | 6.4 ± 4.7 |
| Pain, VAS (0–100) | 63.1 ± 12.8 |
| Disease severity, VAS (0–100) | 64.2 ± 16.5 |
| WOMAC function (0–100) | 56.7 ± 12.8 |
| Symptomatic treatments (% patients) | |
| Analgesics | 80 |
| NSAIDs | 30 |
| SYSADOAs | 35 |
| Radiographic stage of knee OA Kellgren and Lawrence classification, no. of patients | |
| II | 5 |
| III | 9 |
| IV | 6 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




