Abstract
Objective
The goal of the present work was to report two new clinical cases on Charcot spinal arthropathy (Charcot spine) and to identify and review all cases reported in the literature since 1978.
Method
In parallel with a description of the clinical and radiological features of two new cases, we performed a detailed literature review after searching the PubMed and Pascal databases with the following keywords “Charcot spine”, “Charcot spinal arthropathy” and “neuropathic arthropathy of the spine”.
Results
We identified 36 publications comprising a total of 109 cases of Charcot spine. Charcot spinal arthropathy generally occurs as a late complication of traumatic spine injury (17 years afterwards, on average) and predominantly affects the thoracolumbar junction. The main symptoms are pain, spinal deformity, a change in neurological status and the presence of an audible cracking noise during movement. The radiological features combine major disc and vertebral destruction associated with hypertrophic bone formation. The therapeutic indications must be discussed on a case-by-case basis, depending on the patient’s neurological and general status.
Conclusions
Our review emphasizes the value of regular, systematic, long-term radiological and clinical monitoring of the injured spine, in order to detect this rare but probably under-diagnosed complication.
Résumé
Objectif
L’objectif de ce travail est de rapporter deux observations personnelles d’atteinte rachidienne de la neuro-ostéo-arthropathie de Charcot ainsi que de répertorier les cas rapportés dans la littérature depuis 1978.
Méthode
Parallélement à la description des aspects cliniques et radiologiques de nos deux cas originaux, une revue détaillée de la littérature a été réalisée à partir des bases de données Pubmed et Pascal en utilisant les mots clés suivants : Charcot Spine , Charcot spinal arthropathy et neuropathic arthropathy of the spine .
Résultats
Trente-six publications ont finalement été retenues à travers la littérature permettant de regrouper un total de 109 cas de Charcot Spine . L’atteinte rachidienne survient le plus souvent dans un contexte de paraplégie post-traumatique après un délai de 17 ans en moyenne et prédomine au niveau de la charnière thoracolombaire. Les symptômes principaux sont la douleur, la déformation, la modification du statut neurologique et la présence d’un craquement audible. Les aspects radiologiques associent une destruction discocorporéale majeure avec constructions osseuses exubérantes. Les indications thérapeutiques doivent être discutées au cas par cas en fonction du statut neurologique et de l’état général du patient.
Conclusions
Ce travail souligne l’intérêt d’une surveillance radioclinique régulière, systématique et à long terme du rachis des blessés médullaires afin de dépister cette complication rare mais probablement sous-estimée.
1
English version
1.1
Introduction
Charcot spine (also known as Charcot spinal arthropathy and neuropathic spinal arthropathy) consists of rapid spinal joint degeneration following the loss of innervation for any reason .
A causal link between neurological injury and bone/joint damage was first suggested in 1868 by Jean-Martin Charcot, following his observation of the destruction of certain peripheral joints in tertiary syphilis patients ( Fig. 1 ) . Charcot described this damage as “ataxic arthropathy”. However, his publication had been preceded by several observations of limb joint damage in patients with neurological impairments (mainly syphilis patients) .

Although neuropathic arthropathy can affect all the joints (both axial and peripheral), the knee, foot and spine are predominantly affected . Limb damage predominantly affects the legs, except in syringomyelia of the glenohumeral joint . The first report of spinal damage is attributed to the German physician Kronig, who reported a case in a diabetic patient in 1884 .
By definition, neuropathic spinal arthropathy occurs as result of damage to the nervous system. Spinal cord injury is the most frequent trigger and can be due to a wide variety of causes. In years gone by, this was mainly syphilitic tabes dorsalis whereas in recent times, traumatic spinal cord injury is the main cause . However, many other aetiologies are regularly reported (vascular conditions, infectious disease, syringomyelia, tumours, post-radiotherapy conditions, etc.) . The first cases in spinal cord injured patients were described by Slabaugh et al. in 1978 . More rarely, cases have been reported in a context of supraspinal conditions (such as hemiplegia) or peripheral lesions (i.e. peripheral neuropathies, such as in diabetes ). Apart from tabes dorsalis and syringomyelia (the incidence of which is estimated at 5–20% and 25–30%, respectively ), the incidence of the other aetiologies is not known for Charcot spine patients and especially for spinal cord injured patients.
Charcot spine is probably under-diagnosed. Furthermore, diagnosis is often made late in the course of the disease . However, it is essential to recognize this pathology because of its invalidating consequences in spinal cord injured patients (in terms of loss of independence) and the increase in life expectancy when treatment is applied (notably as a result of progress in surgical techniques).
The aim of the present work was to present two new case reports on Charcot spine and to perform a detailed, exhaustive review of the literature. Analysis of clinical cases published in the literature over the last 30 or so years enabled us to perform a quantitative evaluation of some aspects of the disease.
1.2
Materials and methods
1.2.1
New case reports
Here, we report on two clinical cases of Charcot spine in spinal cord injured patients. We provide a detailed description of the occurrence of the condition, the disease history, the clinical presentation, radiological aspects and treatment outcomes.
1.2.2
A detailed literature review
An exhaustive literature review was performed after searching the PubMed and Pascal databases with the following keywords.
“Charcot spine”, “Charcot spinal arthropathy” and “neuropathic arthropathy of the spine”. Only sufficiently detailed studies published between 1978 and 2008 were selected for review. The observations had to include at least the patient’s age, the cause of the neurological impairment and the spinal site affected. Updated studies lacking new case reports were not included in our analysis.
Quantitative variables are described by the mean and standard deviation, whereas qualitative parameters are described by the percentage frequency.
1.3
Results
1.3.1
Case report 1
Our 61-year-old male patient had been suffering from complete ischaemic D10 sensorimotor paraplegia as a result of complications of emergency surgical treatment (at the age of 42) of a ruptured thoracic aorta aneurysm. The ASIA motor score was 50 out of 100, the ASIA light touch sensory score was 69 out of 112 and the ASIA pin-prick sensory score was 68 out of 112 (Frankel A).
At the age of 55, the patient started to complain of increasingly invalidating, chronic pain during movement of the lower lumbar region. This pain was progressively accompanied by major thoracolumbar kyphosis in the sitting position. Clinically, there was no change in neurological parameters or spasticity and no audible cracking sound during movement. We merely noted moderate pain on palpation of the area around the deformation. The patient’s body mass index was about 22. There was no evidence of infection and the laboratory results did not suggest the presence of an inflammatory syndrome.
In view of this radiological evidence, the possibility of a chronic infection or a cancerous process was nevertheless envisaged and prompted us to perform a vertebral needle biopsy. Cultures of central/disc liquid samples were negative. Histological examination revealed the presence of fibrous tissue with sequestered bone but no signs of malignancy. Screening for the HLA B27 gene was negative.
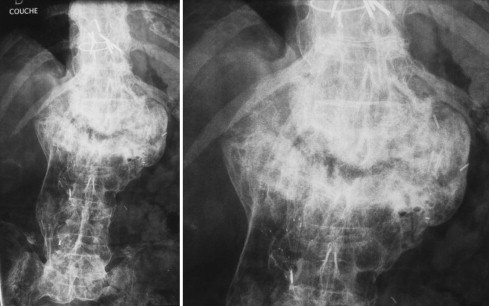
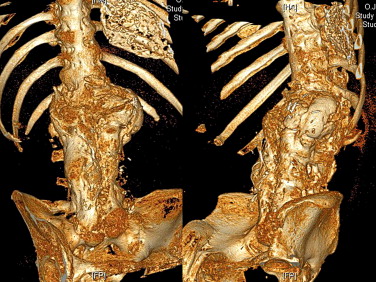
Finally, we arrived at a diagnosis of Charcot spine. Plain X-rays revealed the characteristic aspect of the disease around the L1-L2 joint, with a combination of disc and vertebral destruction, erosion of the L1 and L2 vertebral facets and the presence of paravertebral hypertrophic ossification with a pseudotumoral appearance ( Fig. 2 ). Dynamic X-ray imaging revealed the extent of the spinal instability ( Fig. 3 ) and a three-dimensional computed tomography (CT) scan highlighted the severity of the intervertebral dislocation in the lying position ( Fig. 4 ). The patient was not examined with magnetic resonance imaging (MRI) because he presented a contra-indication (a pacemaker).


In terms of therapeutic options, surgery was ruled out by the patient’s history of cardiovascular disease. Moreover, the extensive spinal ankylosis and widespread para-osteo-arthropathy around both hips ( Fig. 5 ) meant that stabilisation surgery would probably have had a poor functional outcome. External support with a bi-valve body jacket moulded for the sitting position was suggested to the patient but was refused. Hence, we were only able to implement regular clinical and radiological monitoring.

1.3.2
Case report 2
Our second case report concerns a 74-year-old male patient who developed incomplete T10 sensorimotor paraparesis (with leg mobility generally rated at between two and three out of five on the American Academy of Orthopaedic Surgeons motor scale) at the age of 65 following myelopathy due to thoracic canal stenosis and then partial surgical correction. A year previously, the patient had also undergone L2-L5 lumbar laminectomy for the associated degenerative, lumbar stenosis.
At the age of 68 (that is to say around three years after the onset of the neurological impairment), the patient’s condition was marked by significant spinal pain during movement. This worsened progressively over the months, as did the motor impairment. The patient also developed urinary disorders (dysuria and hyperactive bladder).
A clinical examination, in 2001, evidenced aggravation of the impairment with a leg motor scale score of 1 to 2 out of 5, compared with score of 2 to 3 initially. The ASIA motor score was 64 out of 100, the ASIA light touch score was 90 out of 112 and the ASIA pin-prick score was 80 out of 112 (Frankel C). Standing position was not possible. Examination of the legs revealed very low joint amplitudes. The patient’s body mass index of 32 corresponded to moderate obesity.
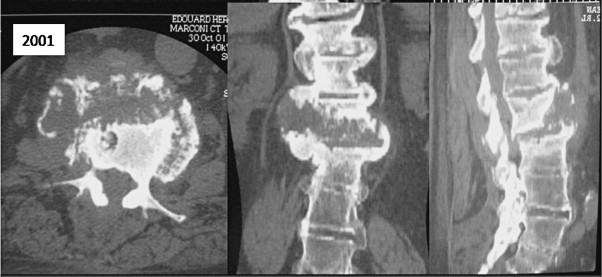
A diagnosis of Charcot spine was initially suggested by the progression of the radiological damage ( Fig. 6 ). In 2007, plain X-rays showed the characteristic appearance of the disease at the L1-L2 joint, with a combination of disc degeneration, vertebral erosion and massive osteophytosis. This damage had developed at the upper extremity of the laminectomized zone. Hence, the time interval between the onset of the paraparesis and the diagnosis of Charcot spine was about 5 years.

A CT scan confirmed the various aspects observed on plain X-rays and provided more detailed information on the paravertebral soft tissue calcification ( Fig. 7 ). The three-dimensional reconstructed images clearly revealed the association of bone destruction and construction phenomena. Furthermore, we noted moderate aggravation of the damage over a 4-year period (between 2001 and 2005) – essentially due to worsening of the L2-L3 retrolisthesis. The patient had not been examined with MRI.
In terms of therapy, the patient continues to refuse any kind of surgery treatment. The combined use of a thoracolumbar body jacket and symptomatic analgesics has helped relieve the patient’s spinal pain (presently three out of 10 on a visual analogue scale).
1.3.3
Literature data
Out of an initial list of 86 articles referenced in the PubMed and Pascal databases, 14 had been published before 1978, 11 described damage to a peripheral joint and seven dealt with Charcot-Marie-Tooth disease. In all, 36 publications were finally selected for review and featured a total of 111 cases of Charcot spine, including our two new case reports ( Table 1 ). Most of the publications reported on only one case ( n = 21 out of 36, i.e. 58,3%) and only four studies reported six or more cases.
| Authors | Year | n | Aetiology |
|---|---|---|---|
| Slabaugh and Smith | 1978 | 1 | Tr |
| Wirth et al. | 1980 | 23 | I ( n = 17), D, M, Tr, Sy, Dia, R |
| Sobel et al. | 1985 | 5 | Tr ( n = 5) |
| Race et al. | 1985 | 1 | Dia |
| Kalen et al. | 1987 | 3 | Tr, I, M |
| Kapila and Lines | 1987 | 1 | Tr |
| Crim et al. | 1988 | 4 | Tr ( n = 4) |
| Piazza et al. | 1988 | 1 | C |
| Mikawa et al. | 1989 | 1 | Tr |
| Schwartz | 1990 | 1 | Tr |
| Luke and Bridwell | 1990 | 1 | M |
| Hoppenfeld et al. | 1990 | 1 | Tu |
| Delvin et al. | 1991 | 10 | Tr ( n = 8), Tu ( n = 2) |
| McBride and Greenberg | 1991 | 4 | Tr ( n = 4) |
| Glennon et al. | 1992 | 3 | A, Tr ( n = 2) |
| Montgomery and McGuire | 1993 | 1 | Tr |
| Pritchard and Coscia | 1993 | 1 | Tr |
| Park et al. | 1994 | 5 | Tr ( n = 5) |
| Heggeness | 1994 | 1 | C |
| Arnold et al. | 1995 | 2 | Tr ( n = 2) |
| Igram et al. | 1996 | 1 | C |
| Standaert et al. | 1997 | 5 | Tr ( n = 5) |
| Thumbikat et al. | 2001 | 1 | Tr |
| Selmi et al. | 2002 | 2 | Tr ( n = 2) |
| Tsirikos et al. | 2004 | 1 | C |
| Mohit et al. | 2005 | 1 | Tr |
| Vialle et al. | 2005 | 9 | Tr ( n = 5), I ( n = 2), Tu, V |
| Rose et al. | 2006 | 1 | M |
| Suda et al. | 2007 | 4 | Tu, Tr ( n = 2), D |
| Staloch and Hatem | 2007 | 1 | Tr |
| Thomason et al. | 2007 | 1 | Tr |
| Cassidy and Shaffer | 2008 | 1 | C |
| Brousse et al. | 2008 | 1 | Tr |
| Sliwa et al. | 2008 | 1 | C |
| Morita et al. | 2008 | 9 | Tr ( n = 6), I, D, R |
| Barrey-Massourides | 2008 | 2 | V, D |
1.3.3.1
Age ( n = 111)
The mean ± standard deviation patient age at the time of Charcot spine diagnosis was 46.7 ± 15.3 (range: 11–77). For the six patients with congenital insensitivity to pain, the age at diagnosis was much lower: 22.3 ± 12 (range: 11–43).
1.3.3.2
Gender ( n = 80)
The series included 63 men and 17 women (i.e. a gender ratio of 3.7).
1.3.3.3
Time to diagnosis ( n = 68)
The mean time lag between the onset of neurological impairment and the diagnosis of Charcot spine was 17.3 ± 10.8 years (range: 1.5–42). This notion of time lag is not applicable to certain aetiologies (a congenital aetiology or diabetes, for example).
1.3.3.4
Vertebra affected by Charcot spine ( n = 163)
The joints affected by Charcot spine are represented in Fig. 8 . We noted a total of 163 affected joints in 111 patients because several patients had damage to more than one joint. The most affected area was L2-L3. We noted the predominance of thoracolumbar and lumbosacral joint damage. The thoracic spine was very rarely affected. In all, 94.5% of the cases of damage were situated below T10 (corresponding to the start of the mobile spine).
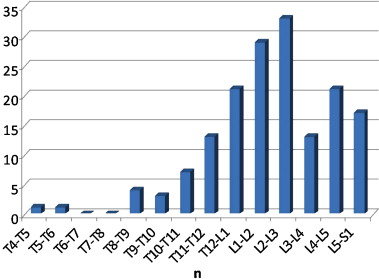
Damage concerned just one spinal joint in most patients ( n = 76 out of 111, i.e. 68.5%), two joints (adjacent or distant) in 25.5% of cases ( n = 28 out of 111) and more than two joints ( n = 7 out of 111) in only 6.3% of cases.
1.3.3.5
The cause of the neurological impairment ( n = 111)
The main aetiology was trauma, accounting for 57.7% of cases ( n = 64 out of 111). Indeed, when considering only the studies published in the last 20 years, a traumatic aetiology was present in over 70% of cases. The infectious aetiologies corresponded primarily to syphilis and became much rarer over time (and totally absent in the most recent publications). All the hereditary causes were due to congenital insensitivity to pain. The inflammatory causes comprised arachnoiditis, three cases of transverse myelitis and a case of ankylosing spondylitis. Miscellaneous causes included one case of syringomyelia, two cases of diabetes and two cases of post-radiotherapy myelitis.
1.3.3.6
Clinical signs ( n = 75)
Only 75 cases were detailed enough to be included in our analysis.
The frequencies of the various clinical signs are summarized in Table 2 . The four most common signs were spinal deformity (essentially kyphosis) and imbalance when sitting, the presence of an audible cracking noise, spinal pain and a change in neurological status. This latter item encompassed a decrease or an increase in the patient’s spasticity, worsening of bladder dysfunction and/or aggravation of sensorimotor impairments of the legs (in incompletely paralyzed patients).
| Symptoms | Frequency (%) |
|---|---|
| Spinal deformity/instability in the sitting position | 46.7 |
| Audible cracking noise | 41.3 |
| Pain | 38.7 |
| Change in neurological status | 38.7 |
| Change in spasticity | 13.3 |
| Sensorimotor impairment/paraesthesia of the legs | 13.3 |
| Aggravation of bladder and bowel disorders | 12.0 |
| Infection | 14.7 |
| Dysautonomic syndrome | 6.7 |
| Other (paravertebral mass, phlebitis) | 2.7 |
1.3.3.7
Radiographic aspects ( n = 61)
Only 61 cases were detailed enough to be included in our analysis. The frequencies of the various features are given in Fig. 9 .

Disc and vertebral damage was always present. Spinal deformity (usually kyphosis) was present in more than 50% of cases and intervertebral dislocation was seen in about 41% of cases. More than a third of the patients (36%) had undergone a biopsy at some point in their medical history; this was usually performed in order to eliminate another cause (either infectious or tumoral) and thus confirm the diagnosis of Charcot spine.
1.3.3.8
Types of treatment ( n = 83)
A sufficiently detailed description of the therapeutic management of Charcot spine was available for 83 patients. Most had undergone surgery (72 out of 83, i.e. 86.7%) with a combined approach (48.6%) or with a posterior approach alone (41.7%). A single, anterior approach had been performed in fewer than 5% of cases and the initial approach was not specified for five patients.
Many patients had already undergone surgery for the initial pathology (particularly in cases of post-trauma spinal damage). The precise boundaries of the initial surgery were known for 50 patients. In over 70% of cases ( n = 36 out of 50), Charcot spine developed within the laminectomized or instrumented zone or at the extremity of the operated zone (often the caudal extremity). However, this was not the case for the patients who had undergone cervical or upper thoracic spinal surgery.
1.4
Discussion
Charcot spine involves the combination of major disc and vertebral degeneration with massive bone formation potentially resulting in dislocation of the spine. On the basis of two new case reports and a systematic and detailed review of 109 cases documented in the literature, we performed a descriptive, comparative analysis of the clinical, radiological and therapeutic data.
1.4.1
The different physiopathological hypotheses
The main disease mechanism in Charcot spine is impairment of joint innervation with loss of proprioception and sensitivity to pain/temperature ( Fig. 10 ). Furthermore, several cases have been described in patients with congenital insensitivity to pain and temperature – an autosomal disease characterized by the absence of small unmyelinated fibres .
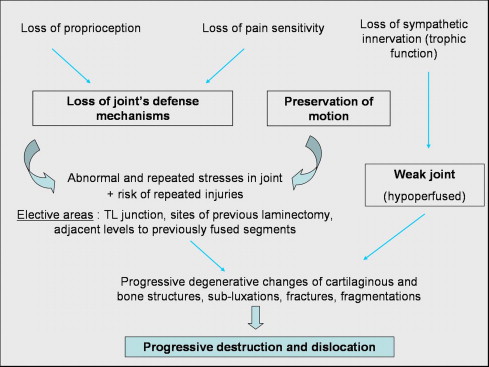
Impaired innervation induces loss of the joint’s defence mechanisms which otherwise help avoid excessive distraction of the disc and ligaments and enable a more homogeneous distribution of the mechanical stresses. Normally, ligament over-extension induces reflex muscle contractions and thus stabilizes the joint .
At the synovial joint, the failure of this reflex process results in repeated microtrauma to the cartilage, leading to progressive destruction of the latter and the joint capsules . Next, one observes changes in the synovial membrane and capsule with hyperplasia and hypertrophy , respectively; this leads to chronic synovial effusion and then progressive sub-dislocation of the joint. The resulting instability triggers the formation of a large osteophyte around the joint. Histological examination reveals non-specific, chronic inflammation and the proliferation of fibroblasts (which replace the joint cartilage) . Destruction of the cartilage leads to osteosclerosis and hypertrophic osteophytosis. Bone necrosis is often observed in peroperative biopsies.
It has also been suggested that loss of sympathetic innervation is a trophic factor that promotes hyperaemia and osteoclast hyperactivity .
Trauma (either repeated microtrauma or a single, major event) appears to be an aggravating factor. In animal experiments performed at the beginning of the 20th century, Eloesser demonstrated the primordial role of trauma in the development of neuro-arthropathy, since loss of joint innervation alone was not a sufficient condition .
In terms of spinal neuro-arthropathy, mechanical factors appear to be critical. In paraplegic patients, spinal stress in the position sitting and during transfers (which repeatedly expose the mobile spine below the fused area to excessive stress) is significant. The risk of early occurrence of Charcot spine is greater in very active spinal cord injured patients . Thus, Charcot spine appears to affect inter-vertebral areas that are exposed to stress (i.e. the thoracolumbar and lumbosacral joints) and/or are fragile.
In previously operated patients, Charcot spine typically develops (in over 70% of cases) either within the operated area (e.g. a non-instrumented laminectomy) or below an extensively instrumented area . According to several authors, laminectomy appears to be an aggravating factor . The loss of posterior stabilizing elements (the interspinous ligaments, the spinous processes, the laminae, the ligamenta flava, the joint capsules) and potential paravertebral muscles damage following laminectomy or, in some cases, partial synovectomy increases the level of stress exerted on the remaining elements (the disc-vertebral body complex at the front and the facet joints at the rear). Six out of the seven laminectomized patients in Vialle’s series developed Charcot spine within the operated zone . Sobel reported similar observations in four out of five cases . In a non-traumatic paraplegic patient, Luke and Bridwell described a case of Charcot spine occurring only 6 months after a laminectomy and a series of rhizotomies performed to reduce spasticity . He thus recommended the performance of a graft and systematic instrumentation in laminectomized paraplegic patients.
1.4.2
Clinical aspects
The time lag between the onset of neurological impairment and a diagnosis of Charcot spine is usually long (17.3 years, on average). In Delvin’s series of 10 cases, the mean time interval was 19 years . The time lag between the first symptoms of Charcot spine and its diagnosis is also long in many cases, due to the relatively non-specific nature of the apparent symptoms.
In paraplegic patients with complete neurologic impairment, the most frequent symptoms are a feeling of instability in the sitting position and spinal deformity (usually with a thoracolumbar gibbosity and an audible cracking noise) . The deformity frequently leads to instability in the sitting position (in four out of 10 cases in Delvin’s series) . In fully paralyzed patients, the presence of spinal pain is frequent; it may be mechanical or inflammatory in nature and is sometimes difficult to differentiate from neuropathic pain. Changes in the neurological picture have also been described: accentuated spasticity in partial paraplegics, decreased spasticity in complete paraplegics and changes in bladder and bowel disorders .
In non-impaired patients or those with incomplete neurological impairment (particularly spinal cord injured patients), the appearance of Charcot spine usually aggravates the neurological status .
It should be noted that a totally unstable spine may be asymptomatic .
In tetraplegic or high paraplegic patients, Charcot spine (typically above T6) can sometimes be revealed by a dysautonomic syndrome that variously combines arterial hypertension, bradycardia, hyperhidrosis and headache . This syndrome can be triggered by certain trunk movements and/or adopting the sitting position . In the literature, surgical stabilization completely resolves the dysautonomic syndrome in such cases.
Lastly (and very rarely), Charcot spine was revealed by a paravertebral, pseudotumoral mass in one case and thrombosis of the left common iliac vein (due to direct compression by an L5 prevertebral bone lesion) in another .
1.4.3
Radiological aspects
The radiological aspects reflect the disease mechanism and usually combine the following features :
- •
significant disc degeneration;
- •
destruction/erosion of the vertebral body that mirrors the disc space, associated with osteosclerosis or osteolysis and, occasionally, the presence of sequestra or debris resulting from fragmentation of the subchondral bone ;
- •
hypertrophic osteophytosis within paravertebral soft tissue, with a markedly pseudotumoral appearance ;
- •
early-onset damage to the facet joints ( Fig. 11 ).
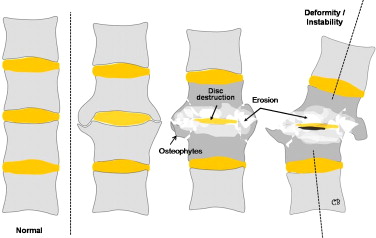
Fig. 11
Schematic diagram of the radiological features of Charcot spine. The cardinal signs are disc destruction, bone erosion (sometimes with fragmentation and debris), disordered paravertebral bone formation and spinal deformity (sometimes leading to true intervertebral dislocation).
As the disease progresses, the damage may take on the radiographic appearance of hypertrophic pseudarthrosis, with the formation of a new intervertebral joint in some cases, so-called ball-and-socket aspect (clinical case 1).
Although the presence of hypertrophic osteophytosis with exostosis is strongly suggestive of Charcot spine, some authors distinguish between a hypertrophic form and an atrophic form in which these giant bone formations are absent and bone destruction phenomena predominate . Delvin found the hypertrophic form in only two of his 10 patients; the remainder had an intermediate form (two cases) or an atrophic form with predominant osteolysis (six cases), . The hypertrophic forms are more often observed around the spine and the proximal joints of the limbs .
Damage to all three columns can induce instability and sub-dislocation (or even full dislocation), leading to kyphotic, scoliotic or combined spinal deformity . Vialle et al. reported the presence of dislocation and/or kyphosis in 90% of cases . Dislocation is present in over 40% of the 111 case reports identified in the present review. This instability can be very significant, as seen in our second case report. Some authors have reported on patients in whom the flexion-extension angle difference is around 60° . In the series described by Morita et al. , the intervertebral mobility observed near the degenerative lesion when comparing the lying and sitting position was 43°, on average 24° to 72°. According to this same study, the severity of the radiological instability correlates with difficulty in maintaining the sitting position. These aspects underline the need to perform dynamic X-ray imaging as part of a patient’s radiological examination.
Furthermore, Morita et al. reported vertebral ankylosing hyperostosis phenomena in seven out of nine patients, with the notable presence of ossification of the anterior longitudinal ligament (as in our first case report).
A CT examination is useful for assessing the severity of vertebral body bone destruction and the extent of paravertebral bone formation. In MRI, one finds frequent peripheral contrast uptake (attesting to the chronic inflammation that accompanies the disc and vertebral damage) and more occasional paravertebral gadolinium uptake . Fluid build-up (seen as hypo-intense areas in T1-weighted images and hyper-intense areas on T2-weighted images) may be found around the paravertebral soft tissues or in the intervertebral space ; this aspect may then suggest spondylodiscitis. The presence of fluid build-up around the arthropathic lesion was seen in eight of Morita et al.’s nine Charcot spine patients (i.e. about 90% of cases) . Furthermore, the authors showed that the fluid volume correlated with extent of the instability seen on X-rays .
1.4.4
Differential diagnoses
The two main differential diagnoses are chronic infectious phenomena and tumoral process . There are no Charcot-spine-specific signs that enable these two diagnoses to be formally ruled out. The following signs are non-discriminant: bone erosion, osteophytosis, narrowing of the intervertebral space and a paravertebral mass . The presence of intervertebral space is strongly suggestive of a degenerative pathology. This explains the high frequency of disc/vertebra biopsies in these patients (36% of cases) with a view to ruling out these differential diagnoses and confirming the diagnosis of Charcot spine. The needle biopsy must be centred on the fluid collection, if the latter is present . The performance of a biopsy appears to be especially justified by the fact that the infection of pre-existing Charcot spine lesions has been reported (14% of the patients in the literature) . It can then be difficult distinguish between post-infected Charcot spine on one hand and primary spondylodiscitis that has induced Charcot-like degenerative lesions on the other. The risk of spinal infection is greater in patients who suffer from recurrent infections (particularly urinary infections). Cases of skin fistula have been reported ( Table 3 ).
| Radiographic signs | Charcot spine | Spondylodiscitis |
|---|---|---|
| Narrowing of the intervertebral space | +++ | +++ |
| Contrast uptake | Peripheral | Central |
| Empty space | ++ | – |
| Osteosclerosis | Vertebral | Limited to the endplates |
| Debris/fragments | +++ | ± |
| Osteophytosis | +++ | ± |
| Facet damage | +++ | – |
Pseudarthrotic fracture of an ankylosed spine is another differential diagnosis ; however, the clinical context and the radiological aspect of the over- and underlying spine enables one (in principle) to differentiate this condition from Charcot spine. Lastly, in patients with a history of spinal surgery, it is sometimes difficult to differentiate a true case of Charcot spine from pseudarthrosis of the original fracture zone or post-laminectomy instability.
Although some authors recommend bone scintigraphy, the technique lacks specificity.
1.4.5
Treatment
Although natural progression of the disease is slow, it is clearly related to aggravation of disc/vertebral lesions . It is also important to emphasize the risk of secondary neurological aggravation in incompletely paraplegic patients . The various therapeutic options consist of monitoring, immobilization with a body jacket and surgery.
Orthopaedic treatment has shown its limitations for medium- and long-term relief and can at best stabilize the disease . It is, however, sometimes the only option for a fragile patient and/or one who refuses any form of surgical intervention . Morita et al. have emphasized the systematic failure of non-surgical treatment alone in cases of infection .
The most radical treatment option is thus surgery , even though some authors consider that the latter is only indicated when orthopaedic treatment fails. Surgical outcomes appear to be better for the spine than for the limbs ; nevertheless, this is a complex surgery, which should only be performed by well-trained teams.
The aim of surgery is to stabilize the diseased spinal segment by obtaining high-quality fusion ( Table 4 ). Debridement of necrotic/inflamed tissue (above all sclerosed and hypovascularized bone) is usually preferable , in order to optimize conditions for bone fusion. The debridement is best performed via an anterior approach . All authors insist on the need to graft the anterior column (the intervertebral space) via either an anterior approach or a posterolateral route (50% of the cases in Vialle’s surgical series). The choice of the exact type of surgery appears above all to be a question of the surgeon’s training and personal preferences.
| Debridement of inflammatory tissues |
| Decompression of spinal canal stenosis |
| Stabilisation |
| 360° bone graft (posterolateral and intervertebral) |
| In 2 stages via a combined approach (posterior + anterior) |
| In 1 stage via a posterior lumbar interbody fusion-type posterior approach, plus a posterolateral approach in some cases |
| Collection of bacteriological and histological samples |
A single, anterior phase is not sufficient in terms of stabilization and runs the risk of mechanical complications . Arnold described the successful operation of two cases of post-trauma Charcot spine via the posterolateral route with decompression, an interbody bone graft and posterior instrumentation . The same was true of the case reported by Mohit et al. (with a T11-T12 interbody cage), . However, most authors recommend stabilization via a combined approach in one or two steps. In 1991, McBride reported the treatment of four cases of Charcot spine with anterior fusion and staged posterior fusion. Only one patient experienced mechanical complications and solid fusion had been obtained in all patients 30 months later . Vialle et al. reported on a series of nine operated patients, with a combined approach applied in eight of these . Fusion was achieved in all cases and resulted in improvements in pain, spasticity and function. In 2007, Suda et al. reported on a series of four cases treated surgically via a combined anterior and posterior approach, with excision of the necrotic and inflammatory tissue, an autologous bone graft and posterior stabilisation with osteosynthesis. The authors noted satisfactory results, with complication-free surgical outcomes, disappearance of the Charcot-related symptoms and successful bone fusion in all four patients.
In order to reduce kyphosis, some authors have suggested performing a posterior shortening osteotomy .
In cases in which bone destruction is limited, a single posterior phase with interbody fusion might be sufficient. Morita et al. notably recommend this strategy in complete sensorimotor paraplegic patients where the risk of neurological aggravation is absent; this approach facilitates surgery which can be awkward if an anterior approach is used . In Morita et al.’s series, bone fusion had occurred in all patients 6 months post-operative.
The boundaries of the instrumented zone (and particularly the lower boundary) are subject to much debate. After fusion, there is a risk of developing damage below the operated spinal segment and above it . In 1992, Brown et al. reported on a series of eight treated surgically cases; two patients experienced mechanical complications and two others developed fresh neuro-osteo-arthropathic damage below the operated zone . Some authors recommend the systematic extension of the operation to the pelvis, in order to eliminating the risk of seeing additional neuro-osteo-arthropathy develop below the instrumentation. However, disadvantages of this approach include:
- •
the complexity of the surgery;
- •
the functional impact in terms of residual mobility when performing transfers.
In a series of 10 cases (with a mean postsurgical follow-up period of 4 years), Delvin et al. obtained fusion in eight cases (80%) and insisted on the fact that no unfused segments should be left between new and old fusions . However, he suggested that extension to the sacrum is not always necessary, even though the risk of seeing a new Charcot spine zone developing between the instrumentation and the pelvis remains. He recommended using a combined anterior and posterior approach for cases of non-reducible, rigid deformity or multiple-level Charcot involvement. For other cases, he performed bone grafting of the anterior defect via a posterolateral approach.
When surgery is used, it is important to evaluate the functional impact of arthrodesis. Morita et al. reported the case of a patient who could no longer perform self-catherization after surgery, due to post-arthrodesis spinal ankylosis. In this respect, the status of the coxofemoral joints must be carefully analyzed, since ankylosis of the hips leads to excessive strain on the lumbar spine; this can result in severe postoperative mechanical complications, such as instrumentation failure and/or pseudarthrosis . An operation to free up the hips can be considered on a case-by-case basis.
Lastly, it is important to take as many peroperative tissue samples as possible, in order to screen for any associated spondylodiscitis or (after histological examination) to rule out a tumour.
1.5
Conclusion
In a systematic review of 36 articles, we were able to identify 111 cases of Charcot spine and describe the main clinical, radiological and therapeutic aspects of this rare but probably under-diagnosed pathology.
If the patient’s health status allows, the optimal treatment is surgical reduction of the deformity and permanent stabilization of the spine. Bone fusion should ideally be achieved by a posterolateral graft and an anterior graft using either a combined approach or an extensive posterior approach alone. Regular postoperative follow-up is also imperative, in view of the risk of delayed damage outside (especially below) the fused segment. However, the indication of surgery must be discussed on a case-by-case basis according to the clinical context (age, the severity of spinal deformation, pain, etc.) as well as the potentially functional impact provided by the surgical procedure.
The present review and the two new clinical cases reported herein also emphasize the value of regular, long-term clinical and radiological follow-up of spinal cord injured patients. This follow-up must examine the entire thoracolumbar spine and not just the site of the initial trauma or pathology.
2
Version française
2.1
Introduction
La neuro-ostéo-arthropathie de Charcot se définit comme un processus dégénératif exagéré au niveau d’une articulation consécutif à une perte de son innervation quelqu’en soit la cause .
Le lien de causalité entre l’atteinte neurologique et l’atteinte ostéo-articulaire a été évoqué pour la première fois en 1868 par Charcot chez des patients atteints de syphilis tertiaire présentant une destruction de certaines articulations périphériques ( Fig. 1 ) . Charcot avait décrit cette atteinte sous le nom d’« arthropathie des ataxiques ». Néanmoins, plusieurs observations d’atteintes articulaires au niveau des membres chez des patients présentant un déficit neurologique, essentiellement des patients syphilitiques, avaient précédé la publication de Charcot .

La neuro-ostéo-arthropathie de Charcot peut toucher toutes les articulations, axiales et périphériques, avec cependant une atteinte préférentielle pour le genou, le pied et le rachis . L’atteinte des membres prédomine largement aux membres inférieurs sauf dans le cadre d’une syringomyélie (atteinte de l’articulation glénohumérale) . Au niveau du rachis, la première description est attribuée au médecin allemand Kronig qui a rapporté un cas en 1884 chez un diabétique .
La neuro-ostéo-arthropathie est, par définition, secondaire à une atteinte du système nerveux. L’atteinte est le plus souvent située à l’étage médullaire et la cause peut être extrêmement variée : essentiellement syphilitique autrefois (tabès) , la lésion médullaire est le plus souvent post-traumatique de nos jours ; mais de nombreuses autres étiologies sont régulièrement rapportées (vasculaire, infectieuse, syringomyélie, tumorale, post-radique…) . Le premier cas chez un paraplégique post-traumatique a été décrit par Slabaugh et Smith en 1978 . Plus rarement, des cas sont décrits dans le cadre de lésions supramédullaires (telle qu’une hémiplégie) ou de lésions périphériques (neuropathies périphériques comme le diabète ). Hormis pour le tabès et la syringomyélie où l’incidence a pu être établie autour de 5 à 20 % et 25 à 30 %, respectivement , le taux d’incidence pour les autres étiologies n’est pas connu y compris chez le blessé médullaire post-traumatique.
Le diagnostic de neuro-ostéo-arthropathie est probablement sous-estimé et le plus souvent établi à un stade avancé de la maladie . Cette pathologie mérite néanmoins d’être reconnue notamment du fait de l’augmentation de la durée de vie des blessés médullaires, des conséquences invalidantes de la maladie en termes de perte d’autonomie pour le patient et des progrès thérapeutiques, en particulier sur le versant des techniques chirurgicales.
Le but de ce travail a été de rapporter deux observations originales de neuro-arthropathie de Charcot localisées au rachis ainsi que d’effectuer une revue exhaustive et détaillée de la littérature. L’analyse des cas cliniques publiés depuis une trentaine d’année à travers la littérature internationale nous a permis d’évaluer de façon quantitative certains aspects de la maladie.
2.2
Matériel et méthode
2.2.1
Cas cliniques
Nous rapportons deux cas cliniques de neuro-ostéo-arthropathie de Charcot avec atteinte rachidienne chez des patients blessés médullaires. Nous détaillons les circonstances de survenue, l’histoire de la maladie, la présentation clinique, les aspects radiologiques et la prise en charge thérapeutique.
2.2.2
Analyse détaillée de la littérature
Une revue exhaustive de la littérature a également été réalisée à partir des bases de données Pubmed et Pascal en utilisant les mots clés suivants : Charcot Spine , Charcot spinal arthropathy et neuropathic arthropathy of the spine . Seules les études suffisamment détaillées et publiées entre 1978 et 2008 ont été retenues. Les observations devaient comprendre au minimum l’âge du patient, la cause du déficit neurologique et le niveau du Charcot. Les études proposant une mise en point mais sans description de cas originaux n’ont pas été inclues dans l’analyse.
Les variables quantitatives sont décrites en termes de moyenne et écart-type alors que les paramètres qualitatifs sont présentés en termes de fréquence.
2.3
Résultats
2.3.1
Cas clinique 1
Il s’agit d’un patient de 61 ans qui présente depuis l’age de 42 ans une paraplégie sensitivomotrice complète de niveau D10, d’origine ischémique, survenue dans les suites du traitement chirurgical en urgence d’une rupture d’un anévrisme de l’aorte thoracique. Le score ASIA moteur était à 50/100 et le score ASIA sensitif à 69/112 pour le tact et 68/112 pour la piqûre (Frankel A).
À partir de l’âge de 55 ans environ, le patient se plaignait de douleurs mécaniques chroniques, de plus en plus invalidantes, au niveau de la région lombaire basse. Ces douleurs s’accompagnaient progressivement d’une cyphose thoracolombaire majeure en position assise. Cliniquement, on ne notait aucune modification de l’examen neurologique, pas de modification de la spasticité, pas de craquement à la mobilisation. On retrouvait simplement une douleur modérée à la palpation en regard de la déformation. L’IMC a été évalué autour de 22. Il n’existait pas de tableau infectieux et le bilan biologique ne mettait pas en évidence de syndrome inflammatoire.
Devant les données du bilan radiographique, un processus infectieux chronique ou tumoral a néanmoins été évoqué et ont conduit à la réalisation d’une ponction-biopsie discovertébrale. Les cultures du liquide centrodiscal étaient stériles et l’examen anatomopathologique retrouva un tissu fibreux avec séquestres osseux sans signes de malignité. La recherche HLA B27 s’est avérée négative.
Finalement, le diagnostic de neuro-ostéo-arthropathie de Charcot était retenu. Les radiographies standards retrouvaient un aspect caractéristique de la maladie au niveau de l’articulation L1-L2 associant une destruction discovertébrale avec érosion corporéale des plateaux des vertèbres L1 et L2 et la présence d’ossifications exubérantes paravertébrales, réalisant un aspect pseudotumoral ( Fig. 2 ). Les clichés dynamiques révélaient l’importance de l’instabilité rachidienne ( Fig. 3 ) et le scanner 3D la sévérité de la dislocation intervertébrale en position couchée ( Fig. 4 ). Le patient n’a pas eu d’IRM puisqu’il présentait une contre-indication (patient porteur d’un pacemaker).