Cancer-related fatigue (CRF) is commonly reported by patients with cancer before, during, and after treatment. It is a persistent sense of tiredness that interferes with function, is distressing, and requires monitoring and, possibly, treatment. Fatigue assessment requires objective measures and self-reports, such as Functional Assessment of Cancer Therapy–Fatigue. Significant contributors to CRF include anemia, pain, insomnia, depressive symptoms, and elevated BMI. Elevated inflammatory cytokines, diabetes mellitus, cortisol, and cellular dysregulation have been associated with CRF. None is causal. Effective treatments include correction of other medical problems, especially anemia, cognitive behavioral therapy, exercise, modafinil, and corticosteroids for short-term use.
Key points
- •
Cancer-related fatigue (CRF) is common, persistent, and difficult to treat.
- •
Causes for CRF are not known, but associations with anemia, high body mass index, diabetes with some tumor types (breast, colon, liver), inflammatory cytokines, insomnia, and cortisol dysregulation are reported.
- •
One of the most effective treatments for CRF is exercise. Aerobic and resistance exercise are both effective.
- •
Cognitive and behavioral therapy mitigate symptoms, as does modafinil.
Background
Cancer and its treatments have been reported to be associated with fatigue in a significant number of patients. Fatigue has been a finding during all phases of treatment, from pretreatment through treatment completion and survivorship. Additionally, it was noted to be associated with significant patient distress. These early reports helped establish an awareness that fatigue was common in patients with cancer diagnoses. What also resulted from these descriptive studies was the belief, later substantiated, that this fatigue was pathologic; that is, persistent and not easily resolved with the usual antidote to fatigue, rest. The early reports proposed that this fatigue was unusual and possibly specific to cancer, in part because its onset was often related to the diagnosis and became almost universal during treatment. Consensus was reached about identifying this as cancer-related fatigue (CRF).
The National Comprehensive Cancer Network (NCCN), a network of practitioners from many disciplines who treat and study patients with cancer, has helped spearhead an effort to bring the needs of patients with cancer into a forum that can help improve and possibly prolong their lives. CRF is one area that NCCN has provided a forum for discussion to raise awareness, and provide data and written guidelines and other tools for management.
NCCN has defined CRF as “an unusual, persistent, subjective sense of tiredness related to cancer or cancer treatment that interferes with usual functioning.” Others have suggested that possibly a better approach might be to use a case definition to describe CRF. This approach suggests 4 criteria to establish the diagnosis: (1) a period of 2 weeks or longer within the preceding month during which significant CRF or diminished energy was experienced each day or almost every day along with additional CRF-related symptoms; (2) the experience of CRF results in significant distress or impairment of function; (3) the presence of clinical evidence suggesting that CRF is a consequence of cancer or cancer therapy; and (4) CRF is not primarily a consequence of a concurrent psychiatric condition, such as major depression. Many agree that CRF is associated with significant distress; interferes with usual activity; is not the result of a psychiatric condition; is likely to be the result of multiple causes, as a result of the disease itself or its treatments; and is difficult to treat.
Because of its prevalence, consensus about the criteria for diagnosis and its impact of function and well-being, CRF has been accepted as a diagnosis in the International Classification of Diseases, 10th Revision (World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision. Version for 2003: http://www.who.int/classifications/icf/en/ ). Many have stated that fatigue is nearly a universal symptom in patients with cancer, mainly during treatment. However, nearly 30% of cancer survivors with no existing disease still have significant fatigue symptoms. Cella and colleagues demonstrated that patients with cancer with anemia, even after successful anemia therapy, and patients with cancer without anemia were significantly more fatigued than controls without either.
In general, CRF is a descriptive term widely accepted by practitioners and patients. The criteria for establishing the diagnosis and its importance as a symptom that needs be followed over time and treated are not questioned. Nonetheless, diagnosis is dependent on a mix of clinical observations and patient self-reports. As of this writing, no metric establishes the diagnosis of CRF and its etiology is not known.
Is CRF a unique form of fatigue? Does it differ from other pathologic fatigue conditions associated with chronic illness? Does it share similar clinical and physiologic findings with chronic fatigue syndrome? In part, the answers to these questions depend on the way fatigue is measured. Improvements in understanding have resulted through the use of operationalized criteria, but classification methods and the validity of diagnostic criteria also are important. Some common findings from other diseases and syndromes may help answer these questions and are discussed in the sections pertaining to clinical presentations and possible biological associations or mechanisms of fatigue.
Background
Cancer and its treatments have been reported to be associated with fatigue in a significant number of patients. Fatigue has been a finding during all phases of treatment, from pretreatment through treatment completion and survivorship. Additionally, it was noted to be associated with significant patient distress. These early reports helped establish an awareness that fatigue was common in patients with cancer diagnoses. What also resulted from these descriptive studies was the belief, later substantiated, that this fatigue was pathologic; that is, persistent and not easily resolved with the usual antidote to fatigue, rest. The early reports proposed that this fatigue was unusual and possibly specific to cancer, in part because its onset was often related to the diagnosis and became almost universal during treatment. Consensus was reached about identifying this as cancer-related fatigue (CRF).
The National Comprehensive Cancer Network (NCCN), a network of practitioners from many disciplines who treat and study patients with cancer, has helped spearhead an effort to bring the needs of patients with cancer into a forum that can help improve and possibly prolong their lives. CRF is one area that NCCN has provided a forum for discussion to raise awareness, and provide data and written guidelines and other tools for management.
NCCN has defined CRF as “an unusual, persistent, subjective sense of tiredness related to cancer or cancer treatment that interferes with usual functioning.” Others have suggested that possibly a better approach might be to use a case definition to describe CRF. This approach suggests 4 criteria to establish the diagnosis: (1) a period of 2 weeks or longer within the preceding month during which significant CRF or diminished energy was experienced each day or almost every day along with additional CRF-related symptoms; (2) the experience of CRF results in significant distress or impairment of function; (3) the presence of clinical evidence suggesting that CRF is a consequence of cancer or cancer therapy; and (4) CRF is not primarily a consequence of a concurrent psychiatric condition, such as major depression. Many agree that CRF is associated with significant distress; interferes with usual activity; is not the result of a psychiatric condition; is likely to be the result of multiple causes, as a result of the disease itself or its treatments; and is difficult to treat.
Because of its prevalence, consensus about the criteria for diagnosis and its impact of function and well-being, CRF has been accepted as a diagnosis in the International Classification of Diseases, 10th Revision (World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision. Version for 2003: http://www.who.int/classifications/icf/en/ ). Many have stated that fatigue is nearly a universal symptom in patients with cancer, mainly during treatment. However, nearly 30% of cancer survivors with no existing disease still have significant fatigue symptoms. Cella and colleagues demonstrated that patients with cancer with anemia, even after successful anemia therapy, and patients with cancer without anemia were significantly more fatigued than controls without either.
In general, CRF is a descriptive term widely accepted by practitioners and patients. The criteria for establishing the diagnosis and its importance as a symptom that needs be followed over time and treated are not questioned. Nonetheless, diagnosis is dependent on a mix of clinical observations and patient self-reports. As of this writing, no metric establishes the diagnosis of CRF and its etiology is not known.
Is CRF a unique form of fatigue? Does it differ from other pathologic fatigue conditions associated with chronic illness? Does it share similar clinical and physiologic findings with chronic fatigue syndrome? In part, the answers to these questions depend on the way fatigue is measured. Improvements in understanding have resulted through the use of operationalized criteria, but classification methods and the validity of diagnostic criteria also are important. Some common findings from other diseases and syndromes may help answer these questions and are discussed in the sections pertaining to clinical presentations and possible biological associations or mechanisms of fatigue.
Clinical presentations
One of the most frequently heard comments from my patients over the decades of treating them is “Why am I so tired? I am exhausted all the time and I am terribly frustrated by this.” My approach has been to enable them to describe their experiences in their own words and hopefully enable me to sort out the contributors to their symptom. I often begin with a thorough evaluation of their medical status and try to identify comorbidities that are treatable, conditions resulting from the cancer and its treatments that might resolve with time, and an overall assessment of the impact fatigue has had on the individual’s life activities. Specifically, it is helpful to ask about the duration, frequency, onset, pattern, and intensity.
Another important, often difficult to quantify issue is the sense of hopefulness and resourcefulness about their disease and its impact. Some have suggested that a patient’s resilience, which correlates well with coping skills, may mitigate treatment-related fatigue. At least one study questions this assumption. In that study, resilience turned out to be a predictor of patients’ fatigue early in the course of receiving radiation therapy. This result is similar to findings from other studies, showing resilience to be a predictor of quality of life and coping in patients with cancer, but it seems to have little influence on treatment-related fatigue for patients receiving radiation therapy.
A list of medical conditions and their association with fatigue is presented in Table 1 , and a list of other conditions associated with fatigue is presented in Box 1 .
| Common | Less Common | Rare |
|---|---|---|
| Anemia Hyper/hypothyroidism Atrial fibrillation Infection Medications (eg, hypnotics, opioids) Depression/anxiety | Renal failure Liver disease Chronic obstructive pulmonary disease/multiple sclerosis Hypercalcemia Arthritis Autoimmune disease Vitamin D deficiency | Adrenal insufficiency Lyme disease Fibromyalgia |
Deconditioning
Inactivity
Insomnia
Stress (physical and emotional)
Dehydration
Nutritional deficiencies
CRF differs from fatigue that is “ordinarily” associated with muscle exertion, delayed-onset muscle soreness, overuse, the flu, or overly exuberant celebration, because it is not relieved by rest or sleep, and does not spontaneously resolve, as following a viral illness. It is, however, entirely consistent that people with CRF may also present with other self-limited fatiguing conditions, such as muscle fatigue or postviral fatigue increasing the intensity in a time-limited way.
CRF has both subjective and objective components and may involve dysfunction in physical performance (physical weakness or tiredness), mood (depression, anxiety), motivation (lack of initiative), cognition (slowing of thought processes, distraction, memory deficits), and social functions (reduced ability to sustain social relationships). All components should be evaluated using proper instruments.
One useful approach to sorting through the presentation of symptoms is to assess whether the individual associates the fatigue with activity or notices that activity exacerbates fatigue to a point thought to be out of proportion to the level of effort. If the latter is an accurate characterization of the fatigue, it is likely to be peripheral fatigue. Peripheral fatigue is usually the result of neuromuscular fatigue and may be the result of depletion of glycogen, sarcolemmal excitability, or the impact of cardiorespiratory limitations. This is thought to be independent of the central nervous system ( Table 2 ).
| Symptoms | Physical Fatigue | Central Fatigue |
|---|---|---|
| Decreased ability to perform activity, decreased exercise tolerance | +++ | +/− |
| Dyspnea on exertion | +++ | + |
| Muscle fatigue/weakness | +++ | − |
| Mood/behavioral change | + | +++ |
| Change in reaction time and attention to task | +/− | + |
| Decline in cognitive performance | +/− | +++ |
| Lack of motivation | +/− | +++ |
If the symptom is present independent of physical activity and is present most if not all of the time, the fatigue is more likely to be related to central fatigue. Central fatigue is a term used to describe a decrement in performance that is not due to a failure in the capacity of muscle to perform. In other words, the muscle has not fatigued to a point where it can no longer contract, suggesting that a mechanism other than local, muscular fatigue is influencing the performance. The explanation is attributed to a central mechanism. One way of measuring this is using a measure of perceived exertion. The most commonly used is the Borg scale.
Central fatigue is the failure to initiate or sustain tasks and activities requiring motivation. The nature of the symptoms associated with central fatigue is difficult to attribute to a specific activity. This type of fatigue has affective components and is frequently experienced by individuals as having no energy, motivation, overall/general fatigue, or changes in mood. This type of fatigue is sometimes equated with not being able to think “clearly” or as rapidly as usual. The term “chemobrain” has been used to link the receipt of anticancer treatment with “fuzzy” thinking. Additionally, people notice memory and recall deficiencies that are often disturbing. It may or may not be associated with depressive symptoms.
Although the distinction between central and peripheral fatigue is a useful framework in which to work, sometimes it is difficult to reliably separate the 2 into distinct entities. One explanation is that individuals select words to describe their feelings and experiences associated with the symptom. Not everyone uses the same words for these. Second, there are more objective measures for physical fatigue determination, and although these provide validity, they do not prove causality. The distinction between peripheral and central fatigue is not universally accepted. Nonetheless, researchers in the field have supported the view that the symptoms can be accurately measured and can distinguish between physical and central fatigue. With the recent interest in brain imaging, and its application to imaging patients with fatigue, pilot data are emerging that suggest there may be abnormal neural network communication in people with persistent fatigue.
There are additional important aspects of fatigue that should be ascertained through patient interviews. These include asking whether the fatigue antedated the diagnosis, or whether it was contemporaneous with treatment (surgery, radiation, chemotherapy, and/or adjuvant). The group whose fatigue is contemporaneous with treatment is less likely to suffer persistent CRF. Data also suggest that migraine, analgesic use, peripheral arterial obstructive disease, and arthritis also are associated with long-term fatigue. Patients who are obese and primarily sedentary have a high risk for CRF. Predictors of long-term CRF are likely to include premorbid depression and fatigue, lack of physical activity, and elevated body mass index (BMI). Other studies have shown similar findings and have included pain as a predictor.
Fatigue measurement
Fatigue management has been targeted as a critical need for cancer survivors that warrants continuous evaluation and treatment. NCCN has issued guidelines for practice with respect to this symptom in patients with cancer ( http://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf ). Among them are that all patients should be screened for fatigue initially and on subsequent visits, treated promptly, and patients and families should be informed that management of fatigue is integral to care for patients with cancer.
This recommendation has been made in part because of the accumulation of significant evidence about the high prevalence of fatigue in patients being treated for and surviving cancer and its adverse impact on life, function, and on patients’ sense of well-being. It is prevalent among all types of cancer, but breast, lung, and pancreatic are among the most frequently associated with persistent fatigue. Some of this may be because patients with breast cancer are among the most frequently studied. Breast and lung are highly prevalent, and breast cancer research has been among the top 3 diagnoses that have received research funds, making it among the most studied cancers, which may partially explain the findings.
Patients and clinicians acknowledge that fatigue is a formidable foe. This is, in part, because there are many possible contributors. Even though we have accepted criteria for diagnosing CRF, not everyone agrees on its cause(s), how to measure it, or how to best mitigate the symptom.
Measuring fatigue has been problematic for researchers studying CRF. One explanation is that most fatigue measures are self-reports, which may not have the precision of objective measures. Nonetheless, significant effort has been mounted to ensure the reliability and validity of these instruments. In fact, seminal work done by a variety of professionals, including nurses, psychometricians, and others has helped develop measurement tools that have placed measurement of patient-reported outcomes (PROs) on a strong methodological footing, demonstrating strong psychometric properties.
Others have reviewed fatigue metrics and recommended a variety of instruments useful for CRF. A recent review of 40 fatigue assessments used to evaluate patients with advanced cancer has found many instruments to be burdensome and therefore favored those that are less complex and with fewer questions. They recommend the Brief Fatigue Inventory and 3 fatigue items of the European Organization for Research and Treatment Quality of Life Questionnaire Fatigue Scale (EORTC QLQ-C30) for assessing patients with advanced cancer. A systematic review of the use and psychometric properties of the multidimensional fatigue symptom inventory short form suggests this instrument has good psychometric properties, clinical utility, and is valid.
In particular, fatigue metrics need discriminant properties to help distinguish between central and peripheral fatigue. Fatigue needs to be distinguished from depression and sleepiness. In reviewing published research for CRF, instruments frequently used are presented in Table 2 . The instruments also need to be sensitive to change so that treatment response can be monitored accurately and the items presented to patients for selection have to align with the kinds of phrases/words likely to be recognized as associated with the fatigue that individuals experience.
Item response theory-based advances in the fatigue measurement have enabled researchers to specifically select questions from an item bank and tailor these to the patient population being studied. Advances in thinking about PROs have led to applying newer technological approaches that use item response theory, including computer adaptive testing. The first steps were to develop a domain framework that focused efforts to organize item pools. The domains are 3 broad areas for self-reports: physical, mental, and social health.
The Patient-Reported Outcomes Measurement Information System (PROMIS) effort has provided options for fatigue and other symptom measurement. One way to use PROMIS is to adopt short forms that have questions preselected based on their link to the constructs being studied. Alternatively, one can select an item bank of one’s own choosing from a larger pool (95 items in the fatigue bank for adults and 23 for children). A recently reported study indicates that PROMIS fatigue measures are responsive to change in 6 different chronic conditions, including cancer.
Another important consideration for measuring fatigue is to be able to identify the ability of the instrument to assess clinically significant findings. Investigators are committed to presenting statistically significant findings, but these should be seen within the context of meaningful differences and clinical relevance. In general, there is consensus about what is meant by clinically meaningful fatigue. Several measures have been evaluated for clinical significance. The SF-36 vitality subscale score of greater than 45 has been shown to be clinically significant fatigue and the Fatigue Severity Index of ≥3.
The other difficulty with respect to fatigue and its measurement is a conceptual one. The word fatigue has many different meanings (connotations and denotations). Using grounded theory, some investigators have been able to identify themes that classify fatigue into physical, affective, and cognitive components. For example, using the Fatigue Assessment Questionnaire, investigators were able to discriminate between fatigue experienced by patients with cancer and controls without cancer diagnoses. A tentative steplike theoretic explanation for the production, perception, and expression of fatigue proposed at the end of one study was supported by factor analysis suggesting that the themes do load separately and can be considered different factors. Other studies have performed factor analysis on a variety of symptoms. One examined symptoms reported by 208 patients with postviral fatigue, and found 4 factors emerged: emotional distress, fatigue, somatic symptoms, and cognitive difficulty. Another analyzed fatigue-related symptoms reported by 780 respondents of persons with chronic fatigue. They found 4 factors: lack of energy, physical exertion, cognitive problems, and fatigue and rest. There remains confusion about these distinctions, and how to determine the different contributors to fatigue. Nonetheless, investigators agree that fatigue is a multidimensional construct.
Measuring physical activity also has been a critical part of the clinical investigation of fatigue for decades. This has been particularly important since the increase in the prevalence of obesity and its apparent connection with sedentary behavior at work and during leisure time. To date, when one speaks of objective measures of fatigue they usually include performance-based measures, such as strength, local muscle endurance, and cardio/respiratory measures of exercise tolerance, such as V o 2 maximum. However, several self-reports of activity have been shown to correlate with these measures. The Human Activity Profile, a self-report of 94 graded questions records what one is able to do, what one can no longer do, and what one never did. The first question asks about one’s ability to arise from a chair (2 metabolic equivalents [mets] of energy) and the 94th asks whether you run 3 miles in 30 minutes (10 mets of work). This questionnaire provides a reliable measure of level of activity and correlates well with aerobic capacity, as does the International Physical Activity Questionnaire. A recent review of the correlations between findings from the self-reported physical activity measures and objective measures, including accelerometers, energy expenditure, and aerobic measures suggest they measure different factors and points out that the basis for the linkage between obesity and lack of physical activity was established using self-reports. Further, that the context of activity, not only its frequency and intensity is important to determine when assessing the activities in which people engage.
Effective management and treatment of CRF symptoms is enhanced when the symptom is well described using qualitative and quantitative measures. The more precisely patients can describe their fatigue and the more accurately it can be measured, the greater the likelihood it can be managed effectively. Meeting these conditions poses a problem because, as described previously, individuals often describe the same symptom differently from each other, and use the same wording to describe different symptoms. Comorbidities (arthritis, diabetes, cardiovascular disease) and clustering of other symptoms (depression, pain, and insomnia) may influence the experience and/or the severity of fatigue, hence these often need to be concurrently evaluated with the fatigue.
One important approach is to provide patients with a careful review of systems and history of prior illness and a comprehensive review of medications. Good control of comorbidities and reduction in medication that may contribute to fatigue should be considered. Medications for control of the primary cancer also are known to cause secondary conditions (eg, arthritis/arthralgia) that associate with fatigue, such as aromatase inhibitors. Once a careful review of systems, determination of comorbidities, and ascertainment of medications are learned, the evaluation should result in a better understanding of the nature of the fatigue, its functional impact, and its possible likely contributors. This will help select targeted, specific treatment.
There has been interest in this concept of clustering of symptoms and efforts to link these in terms of gene expression and proteomics (cytokine profiles). As of now, this has not been shown to be the case. Nonetheless, a unifying theory about mechanism or relationships among these frequently paired symptoms remains an active area for research.
A brief summary of commonly used fatigue scales is presented in Table 3 .
| Name of Instrument | Dimensions of Measurement | CSF Score | Reference | |
|---|---|---|---|---|
| 1 | Fatigue Inventory Scale | Physical, cognitive, and psychosocial fatigue | ≥3 | Fisk |
| 2 | Fatigue Severity Scale | 9 questions, no clear dimensional separate | ≥40 | Krupp |
| 3 | Visual Analog Scale | One dimension | 4 | |
| 4 | Medical Outcome Study Short Form-36 | Vitality subscale | >45 | Ware |
| 5 | FACIT-F | Multidimensional | Yellen | |
| 6 | EORTC | Physical and mental fatigue | Knobel | |
| 7 | PROMIS | Multidimensional, custom questions from item banks | ||
Mechanisms and contributors to fatigue
Inflammation
Cytokines are a group of proteins that communicate between cells. Their effects are protean in that they mediate inflammatory reactions and can stimulate the stress reaction via the hypothalamic-pituitary axis. They either stimulate inflammation (are proinflammatory) or suppress inflammation (are anti-inflammatory). The proinflammatory cytokines, such as interleukins, interferon, and tumor necrosis factors activate corticotropin-releasing hormone. Cytokines and interferon, also used for treatment of melanoma and hepatitis C, have been observed to engender cognitive impairments, mood, and affective changes. Symptoms associated with these treatments also include somatic symptoms, such as headache and fatigue. In part, based on these observations, researchers have sought to investigate the potential role of inflammatory markers in the CRF.
Researchers now need to help explain CRF from the perspective of multiple systems (neuroimmunoregulatory, cardiorespiratory, and musculoskeletal) by creating a biological profile that can connect these systems. Much effort has been spent on assessing associations between inflammatory cytokines as correlates and predictors of fatigue. Studies have documented associations among interleukins, tumor necrosis factors, chemokines, and C-reactive protein in most tumor types. Data support some possible correlations, including the significant rise in plasma interleukin-1 receptor antagonist (IL-1ra) in breast cancer survivors . Elevated levels of IL-1ra, soluble tumor necrosis factor receptor type II (sTNF-RII), neopterin, and soluble IL-6 receptor, have been correlated with fatigue in breast cancer survivors at 5 years after diagnosis. Sleep disruption is associated with CRF, which in turn can be affected by IL-1, IL-6, and TNF-a.
Several longitudinal studies have noted high levels of IL-1ra, and IL-6 during radiation in patients with early-stage breast cancer, as well as elevated IL-1β and IL-6 with stressing stimuli in patients with CRF. The findings are not consistent and when controlled for other variables, age, gender, BMI, and ethnicity, they lose statistical significance. Nonetheless, associations have been reported and have been shown during pretreatment, treatment, and posttreatment phases. This suggests that although the biological insults may differ (tumor, treatment, posttreatment), biological events in patients with cancer diagnoses are likely to have correlations among cytokines and fatigue symptoms.
Several difficulties remain in addition to those mentioned previously. One is that most of these studies are performed in women with breast cancer. Other tumor types are beginning to be reported and share some of the observations about associations with IL-6 and TNF-a, but we await confirmation of the generalizability of the findings. Many of the studies tend to be reported by the same investigative groups and lack confirmatory evidence. Finally, none of the studies has demonstrated a causal relationship between the cytokines and symptom severity or types of fatigue.
Cellular Considerations
Changes in levels of neutrophils and lymphocytes, in particular CD3+, CD4+, and natural killer (NK) cells have been measured in patients with cancer. Similar to the studies in which cytokines and other inflammatory products have been measured, the cross-sectional and longitudinal assessments provide somewhat inconsistent findings. This may be due to the administration of different fatigue metrics, thereby providing a large variance in the severity of fatigue and the lack of control for biological variations, such as BMI. There have been reports of cellular changes following radiation, and indications that the cellular changes persist. Immune dysregulation has been associated with cancer treatment, with a demonstrated decrease in NK cell activity (NKCA). NK cells defend against tumor metastasis, tumor initiation, and tumor growth, and may be of particular importance in epithelial tumors, such as breast cancer. During the early phase after completion of adjuvant radiation therapy, NK cell–mediated antitumor defense becomes particularly important.
Hormonal Factors
The hypothalamic-pituitary-adrenal axis controls the releasing factors that influence thyroid, parathyroid, and adrenal glands and provides cortisol regulation. Cortisol regulation is an important contributor to alterations in glucocorticoid production (including dysregulated circadian profiles) and decreased receptivity of its receptor to hormone attachment. Cortisol is an important regulator of inflammation, both in stimulating demargination of white blood cells and through its anti-inflammatory effects. The role of cortisol has been actively researched as a potential link to the biology of CRF. In other words, it plays an important role in regulating inflammation and in energy production; both phenomena have been identified as correlated with fatigue, suggesting to researchers that there may be a link to metabolic abnormalities, such as seen during the treatment phase of breast cancer, most notably. Reported findings include data suggesting fatigued breast cancer survivors have a significantly more blunted cortisol response to a stressor when compared with those who are not fatigued, even when the frequency of depression is controlled.
Others have found that the phenomenon of blunting the diurnal variation of cortisol occurs only in those who experience physical fatigue. In those patients, the evening cortisol level remains high and the total cortisol secretion is higher than those who do not experience fatigue. Patients with cognitive fatigue do not demonstrate cortisol blunting.
Also reported, and of some interest, is that androgen depletion treatment for men with prostate cancer has accelerated fatigue symptoms during the first year of treatment. This apparently resolves a year into treatment and suggests that significant shifts in circulating levels of androgen may be another contributor to CRF.
Fig. 1 presents a schematic of the neurohormonal interactions thought to be integral to CRF.
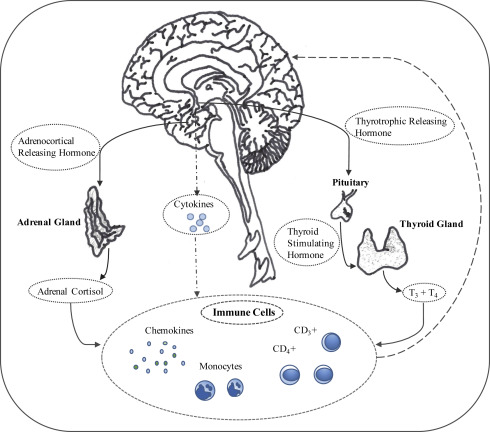
Genetic Factors
During the past decade, investigators have been studying the relationships between gene polymorphisms and symptoms in patients with cancer. This may have begun as an interest in identifying specific tumor signatures so as to target tumor characteristics to maximize therapeutic kill. As gene technological capabilities have progressed, attention has expanded to include unique patient characteristics, to try to maximize the understanding of the host response. This has led to explorations between genetic profiles and cancer-related symptoms.
Most approaches used by these investigators have been to determine whether there are genetic associations between fatigue and various genotypes. The investigators usually select genotypes associated with cytokines that have been shown to be associated with proinflammatory cytokines. Saligan and Kim cite 7 cross-sectional studies in which a variety of genetic findings associated with patterns of fatigue. These included common, homozygous (AA) alleles of IL-6 associating with higher levels of evening and morning fatigue symptoms; higher morning fatigue, but not evening fatigue was with homozygous (GG) alleles of the TNF-α gene; and single nucleotide polymorphisms (SNPs) associated with various cytokines.
SNPs of several cytokines, including IL-1β (rs1143633, rs2853550), IL-1RN (rs397211, rs4252041), and IL-10 (rs1878672, rs3021094) showed significant associations with fatigue levels in lung cancer survivors. The G/G genotype of IL-6-174 and TNF-308; specific catechol-O-methyltransferase (COMT) genotypes (Valine [Val]/Methionine [Met] and Met/Met) were significantly correlated with higher fatigue scores compared with survivors with the Val/Val genotype.
Other descriptive studies report that the presence of specific genetic factors need to be assessed within the context of social and biological milieu, possibly because epigenetic changes may occur. Such factors include that younger, female, unmarried, and black people had more comorbidities, a lower functional status, and were more likely to be in the low morning energy class. Two polymorphisms (IL2 rs1479923 and NFKB1 rs4648110) were associated with this energy descriptive group. Two groups with distinct evening energy trajectories were identified. One is described as younger and male and who had more comorbidities, decreased body weight, and a lower functional status, and were more likely to be in the moderate evening energy class. This group was associated with 5 different polymorphisms (IL1R2 rs4141134, IL6 rs4719714, IL17A rs8193036, NFKB2 rs1056890, and TNFA rs1800683) and had an evening energy pattern. This work points out a very important aspect of fatigue studies that, unfortunately, are not usually addressed. Attending to descriptors of fatigue that differentiate the times of day likely to be “low energy” or more fatigue may be very important to the understanding of fatigue biology. The variable of “when,” not only “how much,” fatigue is one experiencing is not easily measured using standard fatigue instruments. Objective measures of activity are inadequate to determine the details about the symptom.
One issue frequently discussed in the literature reporting genetic associations with cancer-related symptoms is how to sort out the relative contributions of treatment toxicity and tumor effects. In fact, some of the genetic data are questioned with respect to its relevance because of the influence of toxicity. This is a complicated topic and is well reviewed by Vichaya and colleagues.
Based on the number of publications attempting to elucidate relationships between symptoms and genetic profiles of patients, gene expression data, and possible mechanisms for symptom manifestation and control, there is clearly interest in linking biological findings to causal relationships. In my opinion, as of this time, the genetic data provide interesting statistical correlations between symptoms and SNPs, and the biological and demographic information suggest fatigue is a complex symptom and is likely to be influenced by multiple factors and are best considered correlates.
Metabolic Factors
Patients with diabetes are at a higher risk of developing breast cancer than those without either type 1 or 2 diabetes mellitus. Moreover, diabetes is one of the most common comorbidities of breast cancer, at 18%. In one breast cancer survivor study, diabetic patients also obtained higher scores in symptom dimensions, including fatigue, nausea and vomiting, pain, dyspnea, insomnia, constipation, and diarrhea measured by the EORTC QLQ-C30.
Long-term cancer survivors have been reported to have a high prevalence of metabolic syndrome, which poses a significant threat to longevity unless treated. Often the dyslipidemia and glucose intolerance are accompanied by fatigue. Additionally, the survivors most affected are likely to be sedentary. This combination of findings is a significant challenge because it depends on engaging the patients in a long-term commitment to lifestyle changes.
Other Factors
Very robust discussions have appeared in the literature regarding the key features of CRF, in which the similarities and differences lie in comparison with other types of fatigue. These discussions have stimulated research and resulted in statements about the need for continued effort to clarify etiology. Physical fatigue in cancer survivors is frequently associated with inactivity, deconditioning, cardiorespiratory dysfunction secondary to chemotherapy, radiation and/or surgery, and various endocrine and hematological abnormalities. Possible explanations for this have led to explorations about energy production and mitochondrial function at the muscle level. The contributions of local mechanisms, such as contractile properties of muscle, how these contribute to exhaustion, and the central mechanisms of fatigue in the cancer population are currently being actively investigated. Investigators have argued that in the patient with cancer, energy production may have undergone a shift toward the glycolytic pathway, resulting in a greater buildup of lactate and less efficiency of muscle contraction. These abnormalities could possibly link mechanisms of central and peripheral fatigue. Fatigue during prolonged exercise clearly is influenced by a complex interaction between peripheral and central factors. One theory has been that this is a result of central serotonin levels because of its effects on sleep, lethargy and drowsiness, and loss of motivation.
As of the current time, no unifying hypothesis has been convincingly developed to define the mechanism of CRF, despite the considerable literature exploring the chronic fatigue syndrome and its relationship to viral illnesses and neuroinflammation and its relationship to sickness behaviors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







