This article discusses current trends in managing cancer pain, with specific regard to opioid transmission, descending pathway inhabitation, and ways to facilitate the endogenous antinociceptive chemicals in the human body. Various techniques for opioid and nonopioid control of potential pain situations of patients with cancer are discussed. The benefits of using pharmacogenetics to assess the appropriate medications are addressed. Finally, specific treatment of abdominal cancer pain using radiofrequency lesioning is discussed.
Key points
- •
Although the diagnosis of cancer has been stereotyped as a disease to end all, treatment of pain in cancer is showing hopeful prospects.
- •
A strong background in anatomy and physiology is the foundation on which to approach pain in cancer patients, with multiple options accessible.
- •
Use of new medications geared at enhancing the descending inhibitory pathways in combination with classic pharmacologic treatment can improve a patient’s quality of life and reduce side effects.
- •
Reducing pain has been reported to enhance patients’ quality of life and has been associated with longer survival rates.
- •
Being diligent in the reassessment of treatment is the answer to effectively managing complicated cases of cancer pain.
- •
Pain is always changing and, therefore, an astute physician will know how to address the treatment of patients at any time by understanding how pain works.
Introduction
Cancer is a disease that many people prefer not to talk about. Patients with a diagnosis of cancer are met with fear and thoughts of a life-ending illness. During their treatment they suffer significant pain. Various studies have looked at the prevalence of cancer pain, with one study reporting that 50% to 60% of all patients with cancer will experience pain, and other studies reporting a range of anywhere from 19% to 95% of patients with cancer have had or are still having pain. Although studies vary in the reported prevalence of pain in patients with cancer, approximately 70% of patients who die from cancer experience unrelieved pain. Despite national efforts by the Joint Commission on Accreditation of Hospitals and health organizations in the Agency for Healthcare Research and Quality, World Health Organization, and the International Association for the Study of Pain, pain continues to be a significant problem in patients with cancer.
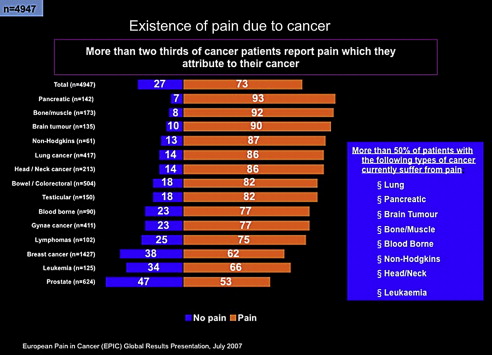
In a European study, the prevalence of pain based on the type of cancer has been well documented ( Fig. 1 ). The most painful cancers appear to be pancreatic, bone, and lung.
In 2011, Marcus and colleagues reported the prevalence of cancer pain to be consistent, with 56% of patients in their study suffering from pain. Thirty-three percent had pain after they underwent treatment, 59% had pain during treatment, and 64% had pain secondary to metastatic disease. All patients suffered functional limitations related to their cancer pain. Patients with head and neck cancer, gynecologic cancer, gastrointestinal cancer, and breast cancer appeared to suffer the most pain. The most consistent barriers to effective treatment of pain were concerns about addiction, cost of therapy, or lack of endorsement by health care providers.
This article discusses pharmacologic management, interventional treatment, and specific cancers that seem to have the highest prevalence of pain, and how interventional treatment and medications can help.
Introduction
Cancer is a disease that many people prefer not to talk about. Patients with a diagnosis of cancer are met with fear and thoughts of a life-ending illness. During their treatment they suffer significant pain. Various studies have looked at the prevalence of cancer pain, with one study reporting that 50% to 60% of all patients with cancer will experience pain, and other studies reporting a range of anywhere from 19% to 95% of patients with cancer have had or are still having pain. Although studies vary in the reported prevalence of pain in patients with cancer, approximately 70% of patients who die from cancer experience unrelieved pain. Despite national efforts by the Joint Commission on Accreditation of Hospitals and health organizations in the Agency for Healthcare Research and Quality, World Health Organization, and the International Association for the Study of Pain, pain continues to be a significant problem in patients with cancer.
In a European study, the prevalence of pain based on the type of cancer has been well documented ( Fig. 1 ). The most painful cancers appear to be pancreatic, bone, and lung.
In 2011, Marcus and colleagues reported the prevalence of cancer pain to be consistent, with 56% of patients in their study suffering from pain. Thirty-three percent had pain after they underwent treatment, 59% had pain during treatment, and 64% had pain secondary to metastatic disease. All patients suffered functional limitations related to their cancer pain. Patients with head and neck cancer, gynecologic cancer, gastrointestinal cancer, and breast cancer appeared to suffer the most pain. The most consistent barriers to effective treatment of pain were concerns about addiction, cost of therapy, or lack of endorsement by health care providers.
This article discusses pharmacologic management, interventional treatment, and specific cancers that seem to have the highest prevalence of pain, and how interventional treatment and medications can help.
Pain in cancer
Treating cancer pain needs to be systematically evaluated. The etiology of cancer pain is related to either direct neoplasm involvement, side effects of chemotherapeutic agents, or radiation-induced plexopathies. The clinician must determine whether the cause of pain is neuropathic, nociceptive, or a combination of both, after which effective treatment can be decided upon.
The OPQRSTU mnemonic will help assess a patient’s pain ( Table 1 ).
| O | Onset | When did it start? Acute or gradual? Pattern since onset? |
| P | Provoking/palliating | What brings it on? What makes it better or worse (eg, rest, medication)? |
| Q | Quality | Identifying neuropathic pain (burning, tingling, numb, itchy, etc) |
| R | Region/radiation | Primary location(s) of pain, radiation pattern(s) |
| S | Severity | Use verbal description and/or 1–10 scale |
| T | Treatment | Current and past treatment; side effects |
| U | Understanding | Meaning of the pain to the sufferer, “total pain” |
| V | Values | Goals and expectations of management for this symptom |
Pharmacologic management of cancer pain
Opioid Therapy
The most common medication used to treat cancer pain is opioids. Although opioids have been used for many years, their efficacy in alleviating cancer pain is questioned. When opioids were discovered, medical knowledge was extremely limited with regard to how pain was transmitted. Early theories attributed pain to only an afferent pathway generating a tissue-injuring message to the spinal dorsal horn. Thus treatment of this pathway for pain consisted of pharmacologic management specific to modulation of components of this sensory message to higher centers. Management consisted of opioid receptor blockers and sodium/potassium channel blockers. Although opioids demonstrated antinociception to the brainstem, forebrain, spinal cord, and the periphery, binding occurred heterogeneously, leading to inadequate treatment of pain.
Descending Pathway Inhibitors
In 1985 Fields and Heinricher demonstrated the presence of a dual-projection system to be highly effective in treating pain through use of the descending pathway.
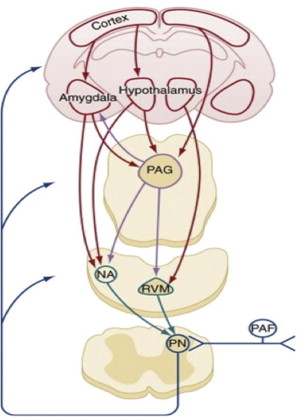
The interrelationship of major neuroanatomic components exerting descending nociceptive control is shown in Fig. 2 . The periaqueductal gray and rostral ventral medulla are strategically located to integrate input from cerebral structures and relay processed information to the spinal dorsal horn. Noradrenergic pontine and medullary nuclei constitute a second important structure directly projecting to the dorsal horn. Presynaptic and postsynaptic mechanisms modulate nociceptive information that is transmitted from primary afferents to spinal projection neurons. Alternatively, indirect actions are exerted via inhibition or excitation of spinal interneurons. Cortical areas, the amygdala and the hypothalamus, are among the cerebral structures exerting top-down control. These structures are relevant for the modulation of pain by stress, emotion, and cognition.
The discovery of a descending inhibitory pathway for pain led to the discovery of various nociceptive neurotransmitters ( Box 1 ). In addition to finding more pain regulators, scientists have also determined how and where these neurotransmitters exert their affect ( Fig. 3 ).
Peripheral neurotransmitters
Hydrogen ions
Norepinephrine
Bradykinin
Histamine
Potassium ions
Prostanoids
Purines
Cytokines (interleukin, tumor necrosis factor)
Serotonin (5-HT)
Neuropeptides
Substance P, calcitonin gene-related peptide, neurokinin A
Leukotrienes
Central neurotransmitters
Glutamate
Neurokinin 1
Substance P
G protein
Neurokinin A
γ-Aminobutyric acid
Calcitonin gene-related peptide
Calcium
Nitric oxide

Fig. 3 illustrates that descending control of spinal nociceptive processes is exerted by multiple neurotransmitters. Transmitters contributing to inhibition of nociceptive signaling are depicted on the left while transmitters enhancing nociceptive signaling are depicted on the right. This physical separation serves didactic reasons and has no anatomic foundation. Transmitters are contained in descending pathways, in inhibitory or excitatory interneurons, and in primary afferent terminals. Only principal transmitter locations are shown, with subtypes indicated in parentheses.
Neuropathic pain does not respond well to opioid therapy and tends to be more problematic. The focus of treating neuropathic pain should be blocking the ascending pathways or increasing the inhibition provided by the descending pathways.
It is theorized that an initiation phase of neuropathic pain is driven by activity from primary afferents, whereas the maintenance phase is mediated by central neuroplastic adaptations. Treatment focused on blocking transmission to the rostral ventromedial medulla (see Fig. 2 ) through lesions or pharmacologic addition of medications that prevent upregulation of spinal dynorphin will help reduce neuropathic pain and neuropathic pain states. Newer medications attempt to work on these descending pathways, on the periphery by modulating GABAergic receptors and centrally by increasing serotonin and norepinephrine. Selective norepinephrine medications such as duloxetine (Cymbalta) are showing promise in treating various forms of pain by enhancing the descending pathways and working centrally on norepinephrine and serotonin receptors and voltage gated calcium-channel blockers such as gabapentin (Neurontin) and pregabalin (Lyrica). Today research is focusing not on opioids but on ways to block these neuropeptides.
NMDA Antagonists
Pharmacology assays in animal behavioral models of neuropathic pain suggest that the N -methyl- d -aspartate (NMDA) receptor is at least partially responsible for facilitated processing, augmentation of painful responses, and subsequent stimuli, but not normal pain sensation. Using NMDA-receptor antagonists such as methadone and buprenorphine can allow titration of analgesic therapy while preventing respiratory depression. Opioid tolerance results in a requirement for increasing doses to achieve a given degree of analgesia. It is thought that continued doses of an opioid will enhance levels of cyclic adenosine monophosphate, protein phosphorylation, and subsequent upregulation of the NMDA-receptor mechanisms within the dorsal horn and supraspinal sites. In addition, accumulation of morphine metabolites may antagonize the analgesic action normally produced by opioid receptor activation. Animal studies indicate that the administration of NMDA antagonists can prevent both the development of tolerance to morphine and the withdrawal syndrome in morphine-dependent rats. Therefore, coadministration of NMDA antagonists such as methadone, buprenorphine, and ketamine with opioids may attenuate the development of opioid tolerance and potentiate opioid analgesic mechanisms. However, the therapeutic window needs to be improved by the use of drug combinations and more selective administration of systemic NMDA-receptor antagonists, and physicians must understand the benefits of NMDA antagonists in comparison with their current use as adjunctive medications. It is hoped that more education on the transmission of pain in medical school, residency, fellowship, and continuing medical education will lead to elimination of the stereotyping of NMDA antagonists.
Cannabis
Various societies are debating the efficacy of cannabinoids in treating pain. Cannabinoid receptors are found throughout the central and peripheral nervous system, and even in the immune system. CB1 receptor tends to predominate in the central nervous system, and CB2 receptors are more extensive in the peripheral and immune systems. Endogenous cannabinoid compounds such as anandamide, 2-arachidonylglycerol, and palmitoylethanolamide act on the CB1 and CB2 receptors to help modulate inflammatory pain and possibly provide analgesic effects. Cannabis sativa L. contains 60 or more cannabinoids; the most prevalent are δ9-tetrahydrocannabinol (THC) and cannabidiol (CB). These 2 cannabinoids seem to mimic the action of endogenous cannabinoid compounds. THC seems to be a partial CB1 and CB2 receptor agonist, thus acting centrally and peripherally, and may produce more psychoactive side effects, whereas cannabidiol seems to act more centrally and produce analgesic and anti-inflammatory effects.
A systemic review of single-dose studies of dronabinol, nabilone, and levonantradol found them to be as effective as 5 to 120 mg of oral codeine. There are also suggestions that cannabis can augment opioid analgesia. Although there is public interest in the utilization of cannabis, more controlled, sizable studies demonstrating true effectiveness are required.
Intrathecal Administration
Intrathecal administration offers an advanced way of delivering opioid therapy with a more direct effect on supraspinal pathways. Although relatively uncommon in patients with cancer, it has some advantages. Patients undergoing intrathecal administration of opioids via an implantable pump ( Figs. 4 and 5 ) will have fewer side effects than those taking oral medications. Owing to the direct effects on the supraspinal pathways, lower doses are required than if used orally. It is generally thought that the concentration ratio from oral to intrathecal is 300:1. A comprehensive review of intrathecal drug-delivery systems is beyond the scope of this article, but certain aspects are highlighted.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







