Bone Densitometry: Using Dual-energy X-ray Absorptiometry for Monitoring
Paul D. Miller
Central dual-energy x-ray absorptiometry (DXA) has been the foundation for monitoring patients with osteoporosis. Monitoring is useful for following the effects of diseases that may negatively affect bone, such as primary hyper-parathyroidism; of drugs that may negatively affect bone, such as glucocorticoids; and of pharmacological agents for the treatment of postmenopausal osteoporosis (PMO).
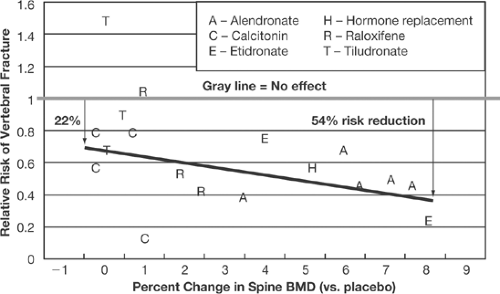
Although there has been widespread acceptance of the use of bone mineral density (BMD) for monitoring disease states or in patients who are taking drugs that may cause bone loss (such as glucocorticoids) [1, 2, 3,4], there has been much debate regarding the utility of BMD to monitor the pharmacological response to osteoporosis therapies [5, 6, 7, 8, 9, 10, 11, 12, 13, 14,15]. The debate has focused on the contribution that pharmacologically mediated changes in BMD make to a reduction in fracture risk. The use of the change in BMD as a surrogate marker for a change in bone strength is clouded by the fact that the increase in BMD mediated by current osteoporosis treatments as it relates to reduction in fracture risk is neither linear nor proportional and by the statistical methods applied to examine the relationship between BMD change and fracture risk reduction. Summary statistics (metaanalysis) [10] (Fig. 4.1) have suggested that this relationship is closer to being linear than the nonlinear relationship defined by individual clinical trial analysis defined by Freedman et al. [14, 15] (Table 4.1). In addition, the U.S. Surgeon General’s report on America’s bone health has stated that surrogate markers can be used within the context of clinical trials to reflect drug-induced improvements in bone strength. Finally, the Food and Drug Administration (FDA) approval of intermittent bisphosphonate dosing intervals (weekly, monthly, quarterly) has been allowed, based on the trust that changes in BMD reflect equal fracture risk reduction as compared with the daily dosing regimens, which were required to prove fracture reduction (“bridging concept”) [16, 17, 18,19]. Nevertheless, other factors lead to fracture risk reduction mediated by osteoporosis-specific pharmacological agents independent of change in BMD [20, 21, 22,23]. In
addition, head-to-head randomized studies, either comparing BMD changes of alendronate to that of risedronate or alendronate to that of teriparatide, have no prospective fracture data to know if differences in BMD increase render a bone stronger [24, 25].
addition, head-to-head randomized studies, either comparing BMD changes of alendronate to that of risedronate or alendronate to that of teriparatide, have no prospective fracture data to know if differences in BMD increase render a bone stronger [24, 25].
Table 4.1. Relationship between pharmacologically induced increases in bone mineral density and fracture risk reduction as assessed by Freedman’s analysis from individual clinical trials | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
Therefore, the debate continues regarding the value of measuring change in DXA-derived BMD as a surrogate marker for fracture risk. This debate, mostly driven by marketing, has clouded the value of BMD monitoring in patients being treated for osteoporosis. Without measuring BMD over time, patients would never receive any feedback to determine if their long-term therapy of an often asymptomatic condition is worth their commitment and
expense. In addition, the discovery of a loss in BMD beyond the in vivo least significant change [26, 27] should never become an acceptable standard of care in the management of the osteoporosis patient on treatment. A loss in BMD cannot be assumed to reflect a residual improvement in bone strength [28, 29]. A loss of BMD may be due to poor patient compliance or adherence to treatment, or to previously unrecognized and possibly reversible secondary conditions that may be responsible for a loss of BMD (e.g., celiac disease). A loss in BMD may be reversed by providing an alternative route of administration (e.g., an intravenous or transdermal or subcutaneous route of administration) or a change in pharmacological agents [30, 31,32].
expense. In addition, the discovery of a loss in BMD beyond the in vivo least significant change [26, 27] should never become an acceptable standard of care in the management of the osteoporosis patient on treatment. A loss in BMD cannot be assumed to reflect a residual improvement in bone strength [28, 29]. A loss of BMD may be due to poor patient compliance or adherence to treatment, or to previously unrecognized and possibly reversible secondary conditions that may be responsible for a loss of BMD (e.g., celiac disease). A loss in BMD may be reversed by providing an alternative route of administration (e.g., an intravenous or transdermal or subcutaneous route of administration) or a change in pharmacological agents [30, 31,32].
Determining the Least Significant Change
The application of the least significant change (LSC) to the practice of DXA use is so important that the International Society for Clinical Densitometry (ISCD) has not only stressed this important in vivo quality performance in their Position Development Conference (PDC) statements but has initiated a nationwide site accreditation process to give recognition to those DXA sites that have performed in vivo precision error studies [33, 34]. Phantoms do not move in serial BMD measurements—patients do. Therefore, the manufacturer-supplied in vitro (phantom) precision error data are not applicable to in vivo quality control studies. To perform an in vivo precision study in order for a DXA site to know its LSC (the minimum change in BMD in absolute or relative terms) and know if a BMD change is significant or a measurement error, a site need only to measure 30 patients in duplicate. This is not research and should not require an IRB (Institutional Review Board) approval, since performing duplicate measurements is often the standard of care in may communities for DXA performance. Once the 30 patients are done in duplicate, the mathematical calculations to find one’s LSC can easily be entered on the ISCD Web site (http://www.iscd.org), which takes just a few minutes to include the patient BMD data to derive a facility’s LSC. Without knowing one’s LSC, one can never know if a change in BMD is real or a measurement error—a fundamental requirement for competent DXA interpretation.
Effect of Anabolic Therapy on Areal Versus Volumetric Bone Mineral Density Measurement
A word should be said about monitoring the effect of teriparatide (recombinant human 1–34 parathyroid hormone). Teriparatide increases bone strength and reduces fracture risk in part by increasing periosteal bone formation [35, 36]. Thus, teriparatide increases bone area. BMD as measured by DXA is a derived equation: BMD = bone mineral content (g)/bone area (cm2) to give a two-dimensional areal measurement (g/cm2). It is possible that patients treated with teriparatide may have a drop in areal BMD yet have an increase in bone strength [37, 38]. In cynomogolus monkeys treated with teriparatide, forearm BMD measured by DXA declined, yet volumetric BMD measured by peripheral quantitative computed tomography increased and forearm bone strength increased as well [36]. The practical issue for patient management is that if the clinician is convinced that the patient being treated with teriparatide is compliant and that secondary factors that might
mitigate a pharmacological response have been excluded, the patient on teriparatide needs reassurance and commitment to continuation of therapy.
mitigate a pharmacological response have been excluded, the patient on teriparatide needs reassurance and commitment to continuation of therapy.
References
Annotations by Elizabeth Dillard
1. Khosla S. Surrogates for fracture end-points in clinical trials. J Bone Miner Res 2003;18:1146–1149.
In this review article, the author discusses the use of bone mineral density (BMD) and bone turnover markers as ways to assess the efficacy of risk reduction in vertebral fracture trials. Traditionally, vertebral fractures were used as endpoints in clinical trials; however, the use of BMD as a surrogate marker could reduce the number of subjects necessary to achieve clinical significance. Models that incorporate changes in both BMD and bone turnover markers could prove to be more accurate predictors of fracture risk reduction.
2. Miller PD. Bone density and markers of bone turnover in predicting fracture risk and how changes in these measures predict fracture risk reduction. Curr Osteoporos Rep 2005;3:103–110.
This review article discusses the assessment of markers such as bone mineral density (BMD) and bone turnover markers (BTM) used to determine the clinical response to pharmacological therapy of osteoporosis. Changes in either measurement can be indicative of reductions in nonvertebral and vertebral fracture risk. An assessment of BTM change earlier in therapy can provide a clinician with information on compliance and the physiologic effects of drug therapy. Increases in BMD after 12 to 24 months also correlates with increase in bone strength, and no change in BMD can be associated with risk reduction in certain clinical trials. Fluctuations in BMD and BTM can be used together to determine efficacy of pharmacological management.
3. Miller PD, Bilezikian JP. Bone densitometry in asymptomatic hyperparathyroidism. J Bone Miner Res 2002;17 (Suppl 2):N98–N102.
4. Miller PD, Hochberg MC, Wehren LE, et al. How useful are measures of BMD and bone turnover? Curr Med Res Opin 2005;4:545–554.




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree