This article reviews the basic principles and therapeutic options in the management of the neurogenic bladder due to multiple sclerosis (MS), written primarily for the non-urology provider. An algorithm for the initial management of the MS patient with lower urinary tract symptoms is provided.
Key points
- •
The goals of treatment of patients with multiple sclerosis (MS) and neurogenic bladders are to preserve renal function, achieve social continence, and to minimize urinary tract complications.
- •
Treatments should be tailored to a patient’s functional capacity.
- •
Initial assessment and treatment of the neurogenic bladder can be done by the primary MS provider.
Introduction
Persons with multiple sclerosis (MS) frequently experience sequelae of the disease in the urinary tract. The severity of the urologic symptoms can be highly variable but generally speaking, the more disabled a person is from MS, the more likely neurologic bladder dysfunction is present. Because individuals do not uniformly feel bothered to the same degree given a particular set of lower urinary tract symptoms, the reporting of bladder symptoms by persons with MS is also highly variable. Quality of life issues notwithstanding, poor bladder management can result in urinary incontinence, urinary tract infections (UTIs) (which may provoke MS exacerbations), kidney and bladder stone formation, and loss of renal function. Thus, proper management of the neurogenic bladder is of utmost importance.
The goals of treatment of patients with MS and neurogenic bladders are to (1) preserve renal function, (2) achieve social continence, and (3) minimize urinary tract complications. This article reviews the basic principles and therapeutic options in the management of the neurogenic bladder due to MS, directed primarily to the nonurology provider. There are several published literature reviews and guidelines on bladder management in MS, and although they contain recurring themes, there is no clear consensus on a treatment algorithm. This article incorporates many of the recommendations, within the context of the author’s extensive experience of providing urologic care for patients with MS.
Introduction
Persons with multiple sclerosis (MS) frequently experience sequelae of the disease in the urinary tract. The severity of the urologic symptoms can be highly variable but generally speaking, the more disabled a person is from MS, the more likely neurologic bladder dysfunction is present. Because individuals do not uniformly feel bothered to the same degree given a particular set of lower urinary tract symptoms, the reporting of bladder symptoms by persons with MS is also highly variable. Quality of life issues notwithstanding, poor bladder management can result in urinary incontinence, urinary tract infections (UTIs) (which may provoke MS exacerbations), kidney and bladder stone formation, and loss of renal function. Thus, proper management of the neurogenic bladder is of utmost importance.
The goals of treatment of patients with MS and neurogenic bladders are to (1) preserve renal function, (2) achieve social continence, and (3) minimize urinary tract complications. This article reviews the basic principles and therapeutic options in the management of the neurogenic bladder due to MS, directed primarily to the nonurology provider. There are several published literature reviews and guidelines on bladder management in MS, and although they contain recurring themes, there is no clear consensus on a treatment algorithm. This article incorporates many of the recommendations, within the context of the author’s extensive experience of providing urologic care for patients with MS.
Lower urinary tract anatomy and physiology
Anatomy
The lower urinary tract is composed of the bladder, which includes the internal sphincter, external sphincter, and urethra ( Fig. 1 ). The bladder is a hollow muscular reservoir within the pelvis. It is composed of interconnecting smooth muscle cells interspersed with elastin and collagen fibers and lined with transitional cell epithelium. The bladder is immediately posterior to the symphysis pubis. The posterior wall and dome of the bladder abut the uterus in women, and in men the ano-rectum is posterior to the bladder. The dome of the bladder is covered by peritoneum, so the bladder is closely related to the sigmoid colon and small intestines. The ureters enter the bladder on its posteroinferior surface. The muscle fibers of the bladder condense into a funnel-like structure at the base forming the bladder neck, also known as the internal sphincter. The structure is not a true circular sphincter, but a thickening of muscle as the bladder transitions into the urethra.
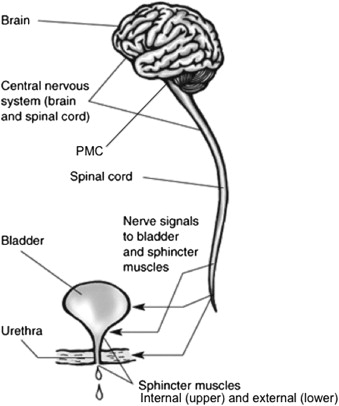
The female urethra is approximately 4 cm long and lies beneath the pubic symphysis, adjoining the anterior vaginal wall. Midway along the urethra is a true sphincter composed of striated muscle, the external sphincter. It is part of the pelvic floor musculature and surrounds the middle third of the urethra in women. In men, the urethra extends out from the internal sphincter and traverses the prostate gland, which is approximately 3 to 4 cm in length. Immediately distal to the prostate gland is the point at which the external sphincter surrounds the male urethra. Past the external sphincter, the urethra continues through the penile shaft to the urinary meatus at the glans penis.
The lower urinary tract is innervated by components of both the somatic and autonomic nervous systems (see Fig. 1 ). The bladder receives its motor innervation through the autonomic pelvic nerves, which are derived from spinal segments S2–S4, with most of the innervation from S3. The bladder neck or internal sphincter is innervated by the hypogastric nerves, which are derived from spinal segments T11-L1. The external sphincter is the only structure in the lower urinary tract to receive somatic motor innervation, and this is mediated through a branch of the perineal nerve, the second segment of the pudendal nerve. Its fibers are derived from spinal segments S2–S4. The sensory fibers for the structures of the lower urinary tract follow the same tracts as their respective motor fibers.
Physiology
Lower urinary tract function is a complex system of reflexes, involving the bladder, internal sphincter, external sphincter, urethra, peripheral nerves, spinal cord, and brain. Neurologic diseases such as multiple sclerosis can result in pathology at any level along the neuraxis and affect the interaction of these reflexes, causing lower urinary tract dysfunction.
In most healthy humans, both kidneys produce urine that drains through the ureters and accumulates in the bladder. The normal function of the bladder is to store urine until it has reached appropriate capacity and is socially acceptable to evacuate. Urine storage is accomplished at low pressures because the bladder wall contains elastin, which allows it to stretch without subsequent increase in pressure. Typical adult bladder capacity is approximately 300 to 500 mL.
The internal sphincter, or bladder neck, remains closed during bladder filling, and the closure is mediated through a constant sympathetic discharge via the hypogastric nerves. Thus, the internal sphincter’s function is purely involuntary. The external sphincter, composed of striated muscle, also maintains a resting tone. It reflexively contracts to maintain continence during periods of increased intra-abdominal pressure and also contracts under voluntary control to shut off the urinary stream or to inhibit urinary urgency.
The detrusor reflex (also known as the micturition reflex) consists of an afferent arm, a central nervous system (CNS) nucleus, and an efferent arm. As the bladder fills, its visceral afferents travel through the pelvic nerves innervating the bladder, ascending through the spinal cord to the pontine micturition center (CNS nucleus) in the midbrain. As bladder volume approaches capacity, the sensation of fullness reaches consciousness, which prompts the individual to seek an appropriate environment in which to urinate. During this time of bladder filling, inhibitory signals from the frontal cortex project on to the pontine micturition center, which prevent the detrusor reflex from occurring. When bladder capacity is reached and is socially acceptable to evacuate urine, the inhibitory signals are withdrawn and a detrusor contraction is initiated at the pontine micturition center. An efferent response travels through the spinal cord and pelvic nerves, and a detrusor contraction occurs. The contraction is coordinated with the opening of the internal and external sphincters to allow free egress of urine from the bladder. A normal detrusor contraction of adequate strength and duration empties the bladder completely in one coordinated and sustained contraction. After urine evacuation, the sphincters return to their closed state and the cycle resumes.
Pathophysiology
Lower urinary tract dysfunction in MS can manifest in one or more of 4 possible physiologic disruptions, depending on the location of neurologic damage or demyelination.
Loss of sensation
If the sensory fibers carrying messages of bladder distention to the CNS are disrupted by demyelination, then the pontine micturition center does not receive adequate stimulus with which to initiate a detrusor contraction. Persons with loss of bladder sensation experience urinary retention or incomplete bladder emptying.
Loss of cortical inhibition of the detrusor reflex
This is the most common manifestation of bladder dysfunction in MS. Because of MS lesions, the inhibition of the detrusor reflex by the frontal cortex is lost or impaired, resulting in bladder contractions with limited volitional control. Patients experience urinary urgency (strong sensation to urinate, typically as the bladder starts to contract), frequency (frequent voiding of small urine volumes), and urge incontinence (urine leak as a result of an inability to inhibit the detrusor reflex). This type of bladder activity is also known as “overactive bladder” and resembles the bladder of a child before toilet training; there is an impaired or absent cortical control over the detrusor reflex.
Inefficient bladder contractility
As described earlier, the healthy bladder empties regularly and efficiently, in a coordinated, sustained contraction. With significant spinal cord disease or injury, the bladder contraction is not of adequate contraction strength or duration to empty the bladder in a single coordination, likely as a result of incoordination with the pontine micturition center. What results is a partially emptied bladder with each void, with varying degrees of urinary retention.
Bladder outlet obstruction
One form of bladder outlet obstruction exclusive to patients with spinal cord disease or injury (including patients with MS) is the loss of coordination between bladder and sphincter activity. If the urinary sphincters, which are closed during filling, do not relax and open before bladder contraction, then there is a functional obstruction to bladder drainage. This incoordination of the sphincters and bladder is known as detrusor-sphincter dyssynergia and results in urinary hesitancy, a sense of incomplete emptying, and urinary retention. With the absence of detrusor-sphincter dyssynergia, women with MS rarely experience bladder outlet obstruction. Men can have obstruction as they age, with an enlarging prostate gland impinging on the bladder neck, causing lower urinary tract symptoms.
Neurogenic bladder management
Principles
There are several principles of bladder management that serve to guide treatment decision making, to reach the goals of renal preservation, achievement of social continence, and minimization of lower urinary tract complications. MS health care providers should keep in mind these general concepts as they develop individualized plans of care with their patients.
- 1.
The bladder should empty regularly and efficiently. This is the primary principle to adhere to, as bladder emptying determines the frequency and severity of lower urinary symptoms and morbidity, which ultimately can affect renal function. The healthy adult bladder in an asymptomatic person empties completely approximately 4 to 8 times every 24 hours, depending on fluid intake. Infrequent and inefficient emptying can result in lower urinary tract symptoms, infections, and incontinence. If a patient cannot spontaneously and efficiently void, the use of catheters can overcome bladder emptying failure.
- 2.
Bladder management should be appropriate to functional capacity and should proceed from the least invasive to most invasive techniques, and least complicated to most complicated. Each patient’s cognitive and physical condition will dictate the optimal method for bladder management. Given the progressive nature of MS, these conditions can change with time, and so bladder management must accommodate those changes. Methods to manage the neurogenic bladder can range from very simple behavioral modifications to extensive surgical reconstruction of the urinary tract. Providers and patients should realize that complex bladder management techniques may not be appropriate for patients who are significantly impaired or for patients who have rapidly progressive disease.
- 3.
Bladder emptying should be independent of caregivers. This is a corollary to the principle discussed earlier. Many patients who are debilitated are unable to empty their bladders spontaneously and thus require catheterization. In an effort to avoid chronic indwelling catheterization, some patients are placed on an intermittent catheterization program, performed by caregivers. Although this program may well decrease the risks of chronic catheterization, it creates an unsustainable management plan, whereby a caregiver is required to be present 24 hours per day. In situations where such care is available, typically the caregivers are not the same personnel from day to day, and the risks of UTIs and urethral injury due to inexperienced caregivers far outweigh the risks of chronic catheterization. If patients are in an environment with limited numbers of caregivers (eg, family members), the burden of care can be overwhelming.
- 4.
Effective bowel management is critical to effective bladder management. Because the innervation to the bladder and urethra neuroanatomically overlap with the innervation of the anorectum, the optimization of bladder management necessarily depends on the optimization of bowel care. Regular, efficient emptying of the rectum minimizes the risk of UTIs, facilitates bladder emptying, and improves quality of life. Bowel function can also be affected by lower urinary tract management. For example, many medications used to treat lower urinary tract symptoms (eg, anticholinergic medications) have constipation as a side effect, and so bowel care should be optimized before starting these medications.
- 5.
There are sex differences in neurogenic bladder management. Although the urinary tract physiology of both women and men are the same, the anatomic differences translate into practical considerations when determining an optimal bladder management plan. For example, women who wear chronic indwelling urethral catheters for many years are at high risk for urethral erosion (which results in urine leak around the catheter) particularly if they are post-menopausal. Women who perform intermittent catheterization must be able to transfer to a toilet and catheterize themselves without being able to visualize their urethras, whereas men are able to catheterize and drain urine into a urinal without necessarily needing to transfer, and they are able to visualize their urethral meatus for self-catheterization. Older men (eg, aged >55–60 years) with urinary symptoms may have an enlarged prostate factoring into their pathophysiology, whereas older women may have pelvic floor complications (eg, bladder prolapse) contributing to theirs.
Techniques
There are a large number of treatment options for the MS patient with neurogenic bladder symptoms, ranging from the simple behavioral modifications to the complex urinary tract reconstruction. The most commonly used methods are outlined in Box 1 and arranged in ascending order with respect to invasiveness (NB, the list does not encompass all possible bladder management options). When considering these options for a particular patient, providers should keep in mind the treatment principles outlined in the last section. The following descriptions of the techniques are meant to provide an overview and not as an instructional guide.





