“Using your own tissues to heal” represents a major health care paradigm change and is one of the most exciting minimally invasive options currently available. Biocellular regenerative therapies are rapidly improving in documentation and cellular analyses and are gaining good safety and efficacy profiles. Once considered purely experimental, they have entered into an accepted, translational period to clinical providers, backed by improving science supporting the basic hypotheses. It is a well-recognized and reported alternative to many traditional medical/surgical interventions.
Key points
- •
Autologous Stem/Stromal Cells and Platelet Concentrates Guided to Targets.
- •
Combination of Cells & PRP concentrates work better than either alone.
- •
Biocellular Combination Is Believed To Facilitate Patient’s Own Wound Healing/Regeneration.
Evolution of cell-based therapies
Over the past decade, great strides have been made in the understanding and potential of targeted cell-based therapies. Starting decades ago, use of an irritant solution to stimulate inflammatory reactions has been replaced in the past few years with transition to injecting various platelet-rich plasma (PRP) concentrates for supporting an effective inflammatory reaction at damaged or degenerative sites. Use of the contained growth factors and signal proteins became recognized as offering a significant improvement in tissue healing responses but seemed limited by incomplete repair while requiring a series (often 4–6) to achieve long-term clinical improvement. Current evolution of combining these trophic growth factors and signal proteins with concentrated undifferentiated cellular/stromal populations seemed like a logical and effective modality, moving into the forefront since 2000. Aesthetic and reconstructive applications led the way, because constant challenges of injury, loss of circulatory capabilities, degenerative, repair, and so forth demanded an optimal approach to regenerative needs. In-depth examination of how the body maintains itself revealed that undesignated cells were integrally important to replacing aging cells (such as skin, hair, bowel lining, and so forth). Early on, fat was not thought of as undergoing such homeostatic mechanisms, because typical mitotic activities were not observed. Now it is recognized that rather than a static number of cells varying only in size, mature adipocytes actually undergo total replacement at a rate of 10% to 20% per year but do so in a different form of cell division known as asymmetric cell division. The ability to have resident precursor cells that are capable of responding to local site signals and the ability of providing a replacement cell of the needed type result in potential replacement cell differentiation, while retaining a single precursor cell type. Without that mechanism eventually there would be an uncontrollable stem/stromal cell population.
With the advent of Food and Drug Administration (FDA)–approved tabletop devices for high platelet concentrations via a closed system, use of a simple blood draw yielded more than 4 to 6 times a patient’s own circulating baselines levels. It has been well shown that the higher the achieved concentrations, the proportionally higher delivery of important factors intrinsically involved in all wound healing and repair.
It has become clear that certain tissue characteristics are most favorable for use in cell-based therapies, including easy and safe access and plentiful autologous stores of a group of cells possessing multipotent potential. Multipotency is important in that such cells have the capability of responding to local signals and possess the ability to transform or replenish signals needed at damaged or diseased sites for repair or regeneration.
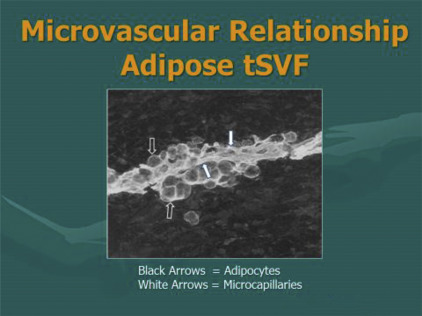
Research has confirmed that a vast majority of such undesignated cells are associated and stored in proximity to the microvascular capillary system ( Fig. 1 ). Essentially all tissues (with blood supply) have some of these multipotent cells available to deal with local and isolated demands. The body retains the ability to chemotactically attract and mobilize cells from local and remote storage points in response to chemical and physical signaling in the body. Approximately 15 years ago, an important scientific advance was made by researchers in finding that adipose tissue (fat) contained high numbers of such cells. This is not totally surprising considering that fat also represents the largest microvascular organ in the body.
Enhancement of cellular and biological therapies comes directly with the ability for providers to be able to identify, target, and guide the cellular-biological combination to areas of injury or degeneration. In that regard, ultrasonography has become a major feature of clinical responses and success. As an example, in medium and deep targets, or those difficult to access, guided musculoskeletal (MSK) ultrasound capabilities offer the optimal integral part of successful responses. Over the past decade, thousands of treatments using biocellular regenerative medicine techniques have proved safe and remarkably effective. The information provided in this article is intended as an introduction to important concepts and describes the current logic believed involved. Major steps have been taken, moving from the laboratory to the bedside. Today, MSK and aesthetic–plastic surgical patients are routinely treated with this combination of biocellular elements.
Evolution of cell-based therapies
Over the past decade, great strides have been made in the understanding and potential of targeted cell-based therapies. Starting decades ago, use of an irritant solution to stimulate inflammatory reactions has been replaced in the past few years with transition to injecting various platelet-rich plasma (PRP) concentrates for supporting an effective inflammatory reaction at damaged or degenerative sites. Use of the contained growth factors and signal proteins became recognized as offering a significant improvement in tissue healing responses but seemed limited by incomplete repair while requiring a series (often 4–6) to achieve long-term clinical improvement. Current evolution of combining these trophic growth factors and signal proteins with concentrated undifferentiated cellular/stromal populations seemed like a logical and effective modality, moving into the forefront since 2000. Aesthetic and reconstructive applications led the way, because constant challenges of injury, loss of circulatory capabilities, degenerative, repair, and so forth demanded an optimal approach to regenerative needs. In-depth examination of how the body maintains itself revealed that undesignated cells were integrally important to replacing aging cells (such as skin, hair, bowel lining, and so forth). Early on, fat was not thought of as undergoing such homeostatic mechanisms, because typical mitotic activities were not observed. Now it is recognized that rather than a static number of cells varying only in size, mature adipocytes actually undergo total replacement at a rate of 10% to 20% per year but do so in a different form of cell division known as asymmetric cell division. The ability to have resident precursor cells that are capable of responding to local site signals and the ability of providing a replacement cell of the needed type result in potential replacement cell differentiation, while retaining a single precursor cell type. Without that mechanism eventually there would be an uncontrollable stem/stromal cell population.
With the advent of Food and Drug Administration (FDA)–approved tabletop devices for high platelet concentrations via a closed system, use of a simple blood draw yielded more than 4 to 6 times a patient’s own circulating baselines levels. It has been well shown that the higher the achieved concentrations, the proportionally higher delivery of important factors intrinsically involved in all wound healing and repair.
It has become clear that certain tissue characteristics are most favorable for use in cell-based therapies, including easy and safe access and plentiful autologous stores of a group of cells possessing multipotent potential. Multipotency is important in that such cells have the capability of responding to local signals and possess the ability to transform or replenish signals needed at damaged or diseased sites for repair or regeneration.
Research has confirmed that a vast majority of such undesignated cells are associated and stored in proximity to the microvascular capillary system ( Fig. 1 ). Essentially all tissues (with blood supply) have some of these multipotent cells available to deal with local and isolated demands. The body retains the ability to chemotactically attract and mobilize cells from local and remote storage points in response to chemical and physical signaling in the body. Approximately 15 years ago, an important scientific advance was made by researchers in finding that adipose tissue (fat) contained high numbers of such cells. This is not totally surprising considering that fat also represents the largest microvascular organ in the body.
Enhancement of cellular and biological therapies comes directly with the ability for providers to be able to identify, target, and guide the cellular-biological combination to areas of injury or degeneration. In that regard, ultrasonography has become a major feature of clinical responses and success. As an example, in medium and deep targets, or those difficult to access, guided musculoskeletal (MSK) ultrasound capabilities offer the optimal integral part of successful responses. Over the past decade, thousands of treatments using biocellular regenerative medicine techniques have proved safe and remarkably effective. The information provided in this article is intended as an introduction to important concepts and describes the current logic believed involved. Major steps have been taken, moving from the laboratory to the bedside. Today, MSK and aesthetic–plastic surgical patients are routinely treated with this combination of biocellular elements.
What is biocellular medicine?
The term, biocellular , refers to the combination of important biological chemicals (such as growth factors, signal proteins, and chemicals important to wound healing) with undesignated cells (often referred to as adult stem/stromal cells) found widely spread within the body and which participate in tissue maintenance, repair, and regeneration. Science and medicine have recently entered a translational phase, where proved laboratory science has demonstrated important contributions join the clinical application of the science in human applications in the past decade. There has been controversy concerning the use of the term, stem cells , in current practice of medicine. Unfortunately, these arguments typically occur with the use of stem cell interpreted as uses of pure embryonic or fetal stem cells, implying destruction of embryo or fetal tissues. In the past decade, the recognition of the safety and efficacy of using a person’s own (autologous) adult stem/stromal cells has advanced to the point that it is widely documented and published ( Box 1 ).
- •
Return to full form or function
- •
Eliminate or markedly decrease pain
- •
Resist recurrence of injury
- •
Reverse, stabilize, resist degeneration
- •
Use autologous tissues for repair
- •
Restore tissues with minimal scar
- •
Accelerate healing processes
Cellular Components
Biocellular regenerative medicine within the United States currently refers to use of autologous, adult (nonembryonic) multipotent cells capable of participating in maintaining tissues (homeostasis), healing, and regeneration. Since 2006, the number of scientific studies demonstrating the values of the highly variable stromal cell populations has exploded, to the point that active reports and studies of component cells of adipose origin exceed the study of nonhematopoietic stromal cells in bone marrow in MSK and aesthetic–plastic surgical applications. The importance of such studies, and an understanding that adipose tissue deposits have gained such recognition due to the greater numbers of stem/stromal cells (other than blood forming element) in the body, is coupled with the important overlap of potential cellular functions. Essentially every tissue in the body that contains microvascular supply maintains a reservoir of such cells. That said, it is recognized that adipose tissues possess the greatest microvascular organ in the body. Many peer-reviewed scientific reports suggest that adipose-derived (AD) stem/stromal cells of mesodermal origin provide between 1000 and 2500 times the actual numbers found in bone marrow. With the easy collection of adipose tissue, less penetration, widely heterogeneous cellular populations, and important immune-privileged properties, subdermal fat deposits serve as a primary source for gathering stem/stromal cells ( Box 2 , Fig. 2 ).
- •
Ease to get
- •
High quantity of cells
- •
Minimum morbidity of donor site
- •
Safety after implantation (own cells)
- •
Multipotent and proliferative cell groups
- •
Secrete immunomodulatory factors
- •
Immunoprivileged cells preferred
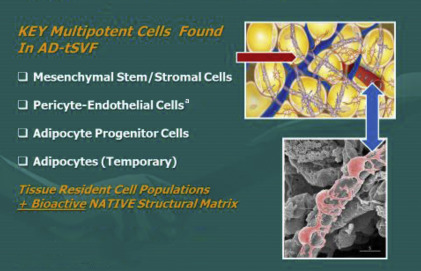
The small nucleated cells found closely associated within the vascular tissues are recognized as serving important roles in maintaining normal tissue content (homeostasis) plus having the ability to respond to injury or disease processes in a constant effort to heal or repair damaged cells (as in aging, arthritis, MSK tissues, neurologic disorders, and so forth). The remarkable design of the human body uses these reservoirs of available, nondifferentiated multipotent cells as the tissue first responders in the situations of major trauma, microtrauma, and aging. By secretion of certain chemicals from an injured site, these multipotent cells (ie, can become various types of cells) can be called on to participate in the repairs needed to restore tissues and functions. There are many peer-reviewed publications that provide examples of how the cells involved in this process can be enhanced by combined provision of the cellular, native scaffolding, and biologically active components ( Box 3 ).
- •
Use your own cells (autologous)
- •
Safe and easy harvest via closed system
- •
Optimal to include native matrix
- •
Transplant in same surgical session
- •
Predictable and reproducible outcomes
- •
Optional ability to use parenteral uses for systemic disorders
- •
Require minimal manipulation (offer optional culture/expansion)
Biological Components
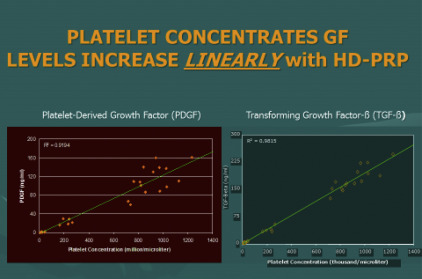
The biological components in this context refer specifically to the availability of a diverse and important variety of growth factors and signal proteins that interact with the cells of degenerative or damaged sites to help recruit needed reparative cells and materials to repair the area. There are 2 major biological components in common use. One is found within recognized contents of platelets, which store and release a wide variety of needed growth factors and proteins to act on available cells to begin the wound healing processes. For many years, the only important role of platelets was believed to become “sticky,” that is, adhere to each other and participate in clotting mechanisms. It is now realized that this may be their least important contribution to wounds and wound healing (with exception of providing a fibrin clot to permit gradual release of platelet contents). Platelets represent a storehouse of small granules, each containing important growth factors and signal proteins that serve to quarterback the healing cascade and do this for a prolonged time during the healing phases. For example, an important chemical available from these granules is essential for blood vessel replacement and repair to improve the circulation ability critical to healing of all wounds. Without adequate blood flow, needed oxygen can neither reach the area of damage nor permit migration of a variety of cells from nearby or distant cell sites ( Figs. 3 and 4 ).
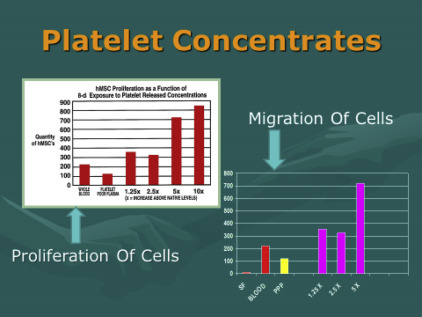
The second source of biological contributors is found in bone marrow aspirates. Bone marrow has been used for many decades, and it is common use in blood-related disorders. Bone marrow does demonstrate microvasculature and therefore does have some undesignated cells (stem/stromal cells). They are, however, in very low numbers compared with adipose tissues. Therefore, many regenerative practitioners consider bone marrow as primarily a valuable biologic and platelet source. To become a valuable cell contributor, it is required that bone marrow aspirates be isolated, concentrated, and culture-expanded to achieve meaningful numbers needed in regenerative and healing applications. This source is technically more invasive to obtain, poses higher complication-sequelae rates, and is significantly more expensive to the patients. In addition to the undesignated stem/stromal cells, content considered of value (such as mesenchymal, periadventitial, and endothelial) is more scarce compared with millions of resident hematopoietic cells. The primary difference between concentrated platelets from peripheral blood versus marrow concentrates is a large store of hematopoietic stem cells (HSCs) while offering an almost identical platelet content. At this time there is little evidence of significant contribution to the platelet regeneration process of MSK tissues derived from the HSC group.
Concentrates primary importance and value is the ability to provide important growth factors and cytokines/chemokines to optimize earlier healing conditions and abilities. Of even more importance in the cellular therapeutic-based effects seems to be their important paracrine secretory influences rather than contributions of individual cellular components and physical engraftment. Furthermore, it well established that the mesenchymal group (MSC) of multipotent cells may originate from the pericyte-endothelial cell groups. Stromal cellular elements offer a great amount of overlapping capabilities in vitro, suggesting that all tissues having some microvasculature and have resident stem/stromal elements capable of providing first responders to sites of damage or degenerative effects. It is suggested that the MSC groups overlap at greater than 95% in their capabilities. Host site interaction with these cells, growth factors, and signal proteins seem to create a complex, heterogeneous precursor population that is considered site specific in many of their responses.
How did biologic and cellular therapeutic concepts evolve?
Aesthetic and plastic surgeons traditionally have dealt with wound healing and scarring issues for many years. During that time, careful study of the processes of homeostasis, remodeling, and repair led to a better understanding of how the body tissues manage to maintain themselves. For many years, the importance of biologics as a derivative part of platelets become appreciated not only for clotting functions but also for the gradual release of critical chemical components essential to the healing processes with individual sites. These biocellular concentrates are thought to immediately begin to participate in secretions capable of site specific repair and regeneration, while local cells begin to actively contribute. In addition to these elements, appreciation of the importance of the native adipose (3-D) scaffolding (matrix) in provision of essential contact points, which serve to encourage microenvironment changes, including cellular proliferation and chemotactic migration, has come to the forefront. Site specificity greatly influences cellular changes within the nondesignated, heterogeneous multipotent populations found in essentially all tissues that have microvasculature. In addition, appreciation of the importance of cellular secretions (paracrine and autocrine) within these undifferentiated cell groups has been reported to be as great, or greater in some instances, as the multipotent cellular differentiation effects.
Once the complex processes of repair and regeneration were examined closely, it became apparent that determination of specific interactions of any single cell or chemical is not able to be determined. At this time, the ability to create an in vitro situation that can clinically duplicate the in vivo microenvironment, making selection of optimal components impossible.
Key adult multipotent cells are found in essentially every tissue and organ in the body. Determination that some of the highest concentrations of these adult stem/stromal cell populations were found within adipose tissue complex (ATC) has led to a major trend shift to more closely evaluate the activities of such tissues and how they can be easily and safely acquired and concentrated for uses in wound healing and repair. Early on, because adipocytes within the ATC were not thought to cell divide, it was assumed that these were static in number and only changed in size according to lipid storage droplets. At this point, it is clear that adipocytes do have a life cycle, replacing all mature adipocytes every 5 to 10 years. Examination of how they accomplish this replacement, via a process of asymmetric cell division, was found that the precursor cell population reacting to secretions from a senescent adipocyte. This replication by cell division results in a replacement immature adipocyte, whereas the other portion retains its precursor form and abilities. This is logical, in that if otherwise, a massive number of precursor cells would accumulate in the tissues ( Box 4 , Fig. 5 ).








