Arthroscopy: Complications of Surgery for Instability
Carlos A. Guanche
Stephen J. Snyder
INTRODUCTION
In the last decade, arthroscopic management of a multitude of orthopaedic problems has become commonplace. One of the most frequent scenarios addressed is glenohumeral instability, a diagnosis fraught with pitfalls. The challenge begins with making the appropriate diagnosis and ends with a likely complex surgical procedure that requires individualized rehabilitation. The incidence of complications arising from the arthroscopic management of instability, however, has not been clearly elucidated in the literature. There are many case reports of various untoward scenarios, yet there is no organized compilation of all the wisdom acquired through these cases.
In one of the earliest attempts to stratify the risks associated with arthroscopic surgery in general, Small (1) documented an overall complication rate of 1% with shoulder arthroscopy. According to his report, the highest rate of complications (5.3%) was from arthroscopic staple capsulorrhaphy. More recently, Curtis et al. (2) have shown an overall rate of complications of 6% for shoulder arthroscopy with some gradation of the procedures from minor to major. In procedures where arthroscopy alone was performed, there was an overall complication rate of 4.5%, with most of those being postoperative stiffness. With procedures that added an open component, the complication rate increased to 6%. More recently, Weber et al. (3) have estimated that there is a 5.8% to 9.5% incidence of complications with shoulder arthroscopy in general. They also allude to the probable increased incidence with more complex procedures.
RECURRENT INSTABILITY
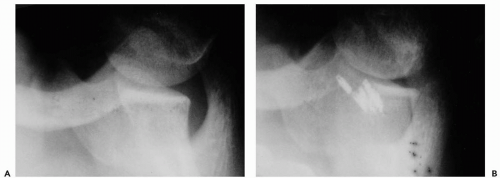
Despite the advances in arthroscopic stabilization, recurrence of instability continues to be the most common complication. The rates of recurrence have varied widely depending on the type of procedure performed. The rates have been documented as 16% to 33% with the use of staple capsulorrhaphy, 0% to 60% with transglenoid suturing, and 0% to 30% with suture anchor repair (4, 5, 6). In some situations, however, no amount of anchoring will maintain stability (Fig. 12-1).
There are many reasons for failure in the reconstruction of the unstable shoulder and these are not necessarily unique to arthroscopic stabilization. They can be divided into a variety of errors attributable to incorrect diagnosis (direction), surgical error, or rehabilitation error. In addition, the episode leading to recurrence is likely to offer some clues as to the etiology. An atraumatic event leading to recurrence in a patient following stabilization may indicate failure to address some component of the instability, whereas a more significant trauma may indicate simple recurrence from a macrotraumatic event. The patient should be questioned with regards to their postoperative satisfaction with the procedure. If the patient indicates that functional
return had not occurred prior to the recurrence event, then they are likely to have undergone inadequate rehabilitation or, in worse cases, sustained a surgical failure.
return had not occurred prior to the recurrence event, then they are likely to have undergone inadequate rehabilitation or, in worse cases, sustained a surgical failure.
 Figure 12-1 Multiple suture anchors following two attempted arthroscopic procedures in a patient with multidirectional instability. |
The most common error made by the surgeon is incomplete diagnosis or, more specifically, failure to address the primary component and/or often the secondary component of instability. This is especially true in cases of multidirectional instability. The reason for this problem is the vagueness of symptoms in most patients. Examination under anesthesia prior to beginning the arthroscopic procedure is critical in that regard. This portion of the procedure is either neglected or not performed at all in many cases. This step should be the final determining factor with respect to any surgical intervention that is undertaken.
Beyond misdiagnosis of the type of instability, failures can be attributable to a lack of understanding of surgical principles. In some situations, a labral detachment is properly addressed but the remaining capsular redundancy is not. This important element of instability correction has historically been described for open procedures, but certainly applies to arthroscopic stabilizations (7). Judicious use of the arthroscope and thorough examination under anesthesia go a long way toward preventing those unfortunate mistakes. A diagnostic arthroscopy should be entertained in any case where there is no significant obvious bony deficiency. Whether the procedure is actually performed arthroscopically or not must be determined by the pathology encountered as well as the arthroscopic skill of the surgeon.
The typical patient will spend 2 hours in the operating room but many days in the therapist’s office. This fact is simply forgotten by many, not the least of whom is the surgeon. To that end, the most common rehabilitation error is that of failure to complete the process (and in some cases, not to institute it at all). A thorough rehabilitation focus beginning with scapulothoracic stabilization and strengthening with a progression to proprioceptive neuro-muscular facilitation is integral to returning the patient to his or her preoperative activity level.
A thorough evaluation of the soft tissues is the key to diagnosing the entire spectrum of injuries that is commonly seen with instability. One area that deserves special attention is the anterior labrum periosteal sleeve avulsion (ALPSA) lesion (8). In this situation, the glenoid labrum, along with the capsule, has been avulsed and then healed in a more medial position on the glenoid neck. This leads to mechanical incompetence of the labrum’s stabilizing effect. This lesion is often missed because it may not be recognized on magnetic resonance imaging (MRI) or it may not be seen from the standard posterior portal.
Other variants in the spectrum of pathologies are the humeral avulsion of the glenohumeral ligament (HAGL) and reverse humeral avulsion of the glenohumeral ligaments (RHAGL) lesions. These rare anterior or posterior ligament detachments have been recognized for many years since first described by Nicola in 1942 (9). However, despite being known, they are often also difficult to identify on imaging studies or arthroscopic evaluation. HAGL or RHAGL lesions are commonly seen after failure of the original stabilization procedure, as has been noted by Savoie (10). Finally, the rotator interval capsule is a complex and poorly understood portion of the anatomy. Although insufficiency of this area contributes to instability in several directions (11), there is a paucity of guidelines for the treatment of rotator interval pathology.
Mologne et al. (12) have analyzed 20 patients that had failed arthroscopic stabilization procedures. In their study (in which the patients were revised with open surgery), they found that 40% of the patients had an unhealed Bankart lesion and 75% were found to have redundant anterior capsules (12). They concluded that capsular laxity is difficult to quantify arthroscopically and is present in a significant percentage of patients with chronic traumatic shoulder instability.
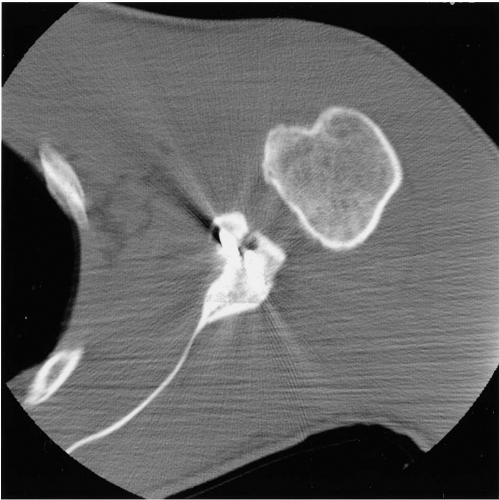
Bony deficiency is another area that warrants careful consideration (13). Historically, the focus on arthroscopic repairs has centered on the reattachment of soft tissue to bone, while ignoring the bony deficiencies. In situations where multiple episodes of instability have occurred, patients may develop a significant bony defect (Fig. 12-2). This is particularly important on the glenoid side, but can also be seen in the form of a large Hill-Sachs lesion of the humeral head. Burkhart and DeBeer (13) have documented that their arthroscopic stabilization patients are more likely to have instability recur when an insufficient glenoid surface was found intraoperatively. In their study of 21 patients having recurrent instability following arthroscopic suture anchor fixation, 14 had sizeable bone defects, with 3
engaging Hill-Sachs lesions and 11 “inverted-pear” glenoid defects.
engaging Hill-Sachs lesions and 11 “inverted-pear” glenoid defects.
The diagnosis of a significant glenoid deficiency can be made radiographically with the use of the Bernageau or glenoid profile view (Fig. 12-2) (14). Confirmation of the deficiency can also be made arthroscopically by measuring the size of the glenoid from the central bare spot to the anterior and posterior edges of the labrum. A size difference of more than 2.5 mm between the two measurements is thought to be indicative of a relatively deficient glenoid (15). Most commonly, these cases are corrected with either a Laterjet-type coracoid transfer procedure (Fig. 12-3) (13), or reconstruction of the glenoid using iliac crest bone graft (16).
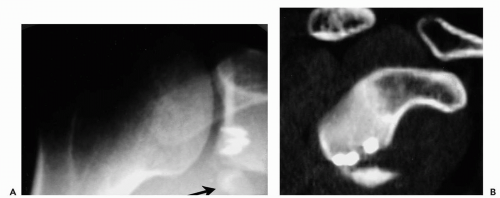
In some situations, a substantial avulsion fracture of the glenoid is either neglected or not addressed at the time of the initial surgical procedure (Fig. 12-4). In these cases, a solid labral repair may still fail as a result of the lack of surface for articulation of the humeral head and the glenoid. Although the etiology of the glenoid deficiency is an acute defect, the same treatment principles apply. The initial surgical approach should address the avulsion and an attempt should be made to reapproximate the bony fragment in as near an anatomical position as possible.
The engaging Hill-Sachs lesion should also be ruled out as a source of recurrent instability. The engaging lesion is defined as a large posterior-lateral humeral head compression fracture situated with its long axis parallel to the anterior glenoid with the shoulder in a functional position of abduction and external rotation. In this location, the Hill-Sachs defect engages the corner of the glenoid (13). If an engaging lesion is documented, the surgery should involve not only a labral repair, but also a restriction of external rotation by capsular plication (performed either open or arthroscopically). Alternatively, the humeral head defect can be extended by filling it with either autogenous or allograft bone (17). A rotational proximal humeral osteotomy can also be performed in severe cases (18). This operation, however, is limited to the most severe cases, often where other measures have failed.
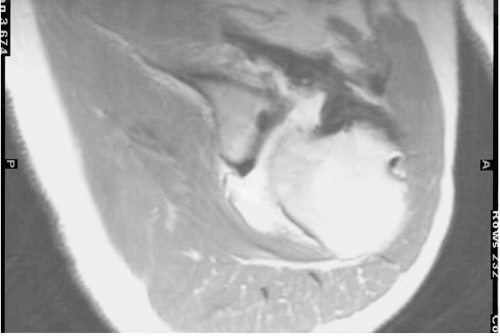
Another area that has received little attention is the lack of healing ability. This is particularly true in older patients where an instability episode is followed by surgical reconstruction. Intraoperatively, the tissue available for repair may be friable or atrophic and minimal trauma may result in a recurrence of the instability, causing a retear of the
labral repair (Fig. 12-5). This is a particularly troublesome situation that has no easy solution, although some attempts have been made to quantify the healing ability based on the appearance of the perilabral tissues (19). The referenced study analyzed the issue relating to the arthroscopic transglenoid technique, the principles of which apply to both arthroscopic reconstruction and open reconstruction. In these cases, open repairs may be more appropriate if the tissue mobilization that is necessary to obtain a solid repair cannot be performed arthroscopically. This concept has especially significant implications with respect to the surgeon’s individual abilities in performing arthroscopic mobilization.
labral repair (Fig. 12-5). This is a particularly troublesome situation that has no easy solution, although some attempts have been made to quantify the healing ability based on the appearance of the perilabral tissues (19). The referenced study analyzed the issue relating to the arthroscopic transglenoid technique, the principles of which apply to both arthroscopic reconstruction and open reconstruction. In these cases, open repairs may be more appropriate if the tissue mobilization that is necessary to obtain a solid repair cannot be performed arthroscopically. This concept has especially significant implications with respect to the surgeon’s individual abilities in performing arthroscopic mobilization.
In summary, although the pathological areas to consider in revision surgery are many, a few principles can be applied to guide one through the process. In general, the arthroscope is a useful tool to delineate the extent of the pathology involved. In some cases, the arthroscopic visualization of the pathology is more readily visible than when assessed in an open fashion. An excellent example would be a HAGL lesion.
In cases where severe bony deficiency is suspected, plain films should serve as a guide for the need for further diagnostic studies, such as a computed tomography (CT) scan. Where the history and radiographs are questionable with regards to the remaining bone stock, a reasonable option is to perform a diagnostic arthroscopy and be prepared to perform an open reconstruction (such as a Laterjet) at the same sitting.
In situations where a severe bony deficiency is present, the arthroscope is not helpful in most cases. In these cases, a single open procedure is typically indicated without the need for a diagnostic arthroscopy. That being said, however, in cases where additional pathology is suspected (such as a rotator cuff deficiency), a diagnostic arthroscopy can help plan the appropriate procedure(s).
LOSS OF MOTION WITHOUT ARTHROSIS
Although the major goal in the treatment of recurrent instability is the restoration of stability and the maintenance of pain-free motion, in some cases excessive tightening results in significant and often disabling painful limitation of motion. Excessive loss of motion, most often external rotation, has been implicated as a cause of degenerative arthrosis (20, 21, 22).
Historically, several open procedures sought to limit the incidence of dislocation and subluxation episodes by intentionally
restricting motion. To date, this has not been a significant source of concern with respect to arthroscopic repairs. Arthroscopic repairs generally tend to recreate the normal anatomy, especially as it concerns a reattachment of the detached capsulolabral complex. It is technically more difficult to perform significant capsular shifts and imbrications as has been described and documented with open procedures. As a result, the more likely scenario following arthroscopic procedures has involved the recurrence of dislocation or subluxation episodes rather than excessive tightness. When stiffness does occur, it is often associated with hardware complications resulting from prominent suture anchors or degradative products of absorbable devices (23, 24, 25, 26, 27).
restricting motion. To date, this has not been a significant source of concern with respect to arthroscopic repairs. Arthroscopic repairs generally tend to recreate the normal anatomy, especially as it concerns a reattachment of the detached capsulolabral complex. It is technically more difficult to perform significant capsular shifts and imbrications as has been described and documented with open procedures. As a result, the more likely scenario following arthroscopic procedures has involved the recurrence of dislocation or subluxation episodes rather than excessive tightness. When stiffness does occur, it is often associated with hardware complications resulting from prominent suture anchors or degradative products of absorbable devices (23, 24, 25, 26, 27).
In summary, it is important to realize that the anatomical reconstruction of the joint is integral to a good outcome. Positioning of suture anchors appropriately on the articular margin is important, as is tightening the capsuloligamentous complex to its original configuration and tension. Advancing the tissue too far may cause an excessive restriction of motion and subsequent stiffness in the joint. When performing arthroscopic capsular closure procedures, it is important to avoid suturing and thus overtightening specific anatomic structures such as the middle or superior glenohumeral ligaments, unless the specific motion restrictions associated with their tightening is desired (11). This is especially dangerous in cases where a rotator interval closure is performed or when the cordlike middle ligament of a Buford complex is mistakenly fixed to the glenoid.
In a patient with restricted range of motion, one must rule out other pathologic entities such as reflex sympathetic dystrophy, rotator cuff tendinitis, inflammatory and crystalline arthropathy, avascular necrosis, secondary gain and psychological dysfunction. In addition, the patient’s surgical procedure, postoperative immobilization, and rehabilitation regimen should all be critically analyzed. The causes of stiffness may be either capsular or extracapsular and a careful examination of the surrounding musculature is important in this respect (28).
ARTHROSIS
Although extremely rare in arthroscopic procedures, patients who have severe arthrosis following an arthroscopic reconstruction may require prosthetic replacement with careful attention to soft-tissue balancing. Long-standing contractures tend to force the humeral head posteriorly as a result of the anterior capsular restriction that tends to develop. This can result in a fixed posterior subluxation with significant posterior glenoid erosion (29).
There are two important considerations when treating a poststabilization arthritic shoulder with shoulder replacement: significant posterior capsular redundancy and posterior glenoid wear. Both problems can contribute to a difficult reconstructive procedure. Careful preoperative planning must address the likelihood of the need to perform a posterior capsulorrhaphy. This may require either an anterior incision or a separate posterior surgical approach. In more severe cases, a glenoid bony reconstruction may be warranted (20).
HARDWARE/IMPLANT-RELATED
Implants placed arthroscopically have not been reported to cause life-threatening hardware migration as have implants placed in open procedures (30,31). It must be assumed that these implants will behave the same as when they are poorly placed in open surgery (Fig. 12-6).
In the early reports of transglenoid suturing techniques, fistula and cyst formation were documented (32) as well as intrathoracic penetration with a Beath pin (33). This type of procedure is no longer employed and the documentation of any of associated complications is largely historical.
The use of absorbable tack devices is relatively commonplace in arthroscopic labral repairs. The biological process of degradation of these devices has been elucidated in scientific literature and it is important to review the process to understand why failures occur. The polymers that are used to fabricate these devices include polydioxanone (PDS) and either combinations or racemic mixtures of polyglycolic acid (PGA) or polylactic acid (PLA).
Degradation of PLA and PGA occurs in a continuum of five stages (34). Initially, hydrolysis from local tissue fluids occurs. The hematoma from the surgery then leads to a fibrous capsule forming with granulation tissue and giant cells. The next stage is depolymerization, which begins with a loss of strength of the device. The kinetics of this mechanism depends on the material’s physical qualities, surface area, and the environment to which it is exposed. The third
stage involves loss of mass integrity as the implant begins to fragment. At this point a mild inflammatory reaction may occur, although this varies with the actual material employed. The fourth stage, absorption, is where hydrolysis allows for phagocytosis and dissolution. Finally, elimination allows for the monomers to be broken down and excreted primarily as carbon dioxide and water from the lungs (PLA/PGA) or via the urinary system (PDS).
stage involves loss of mass integrity as the implant begins to fragment. At this point a mild inflammatory reaction may occur, although this varies with the actual material employed. The fourth stage, absorption, is where hydrolysis allows for phagocytosis and dissolution. Finally, elimination allows for the monomers to be broken down and excreted primarily as carbon dioxide and water from the lungs (PLA/PGA) or via the urinary system (PDS).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree