Arthroscopic Management of the Stiff Shoulder
Thomas M. Lawrence
Scott P. Steinmann
INTRODUCTION
Shoulder stiffness is a common yet challenging clinical disorder presenting to the orthopedic surgeon with multiple etiologies. There is a poor understanding of this problem due to the lack of consensus in defining and differentiating causation, unclear pathogenesis, and natural history, as well as controversy in treatment options. This chapter focuses on the arthroscopic management of the stiff shoulder.
Shoulder stiffness is frequently generically and interchangeably referred to as frozen shoulder or adhesive capsulitis. It can be broadly classified into primary or idiopathic in nature or secondary stiffness where a cause can be identified. It is important to distinguish the etiology of shoulder stiffness as treatment strategies may differ, although difficulties may arise due to overlap and coexisting pathology such as rotator cuff tears.111 Primary shoulder frozen shoulder or adhesive capsulitis is a painful condition that affects between 2% and 5% of the population.44 It is characterized by progressive loss of both active and passive range of motion due to fibrosis and contracture of the joint capsule. Codman22 initially coined the term “frozen shoulder” describing the condition “difficult to define, difficult to treat and difficult to explain from the point of view of pathology.” Neviaser demonstrated the essential pathology to be a “thickening and contracture of the capsule which becomes adherent to the humeral head” and on this basis suggested the term “adhesive capsulitis”.67 Primary adhesive capsulitis typically presents in patients between 40 and 60 years of age, is more common in females and may affect both shoulders, at different times, in up to 30% of patients.8,44 The etiology remains unclear although it has been associated with a number of systemic conditions, including diabetes,62,64,97 thyroid disease,11,92,107 cardiac disease and surgery,99 hyperlipidemia,13 Parkinson’s disease,21 and Dupuytren’s contractures.90
The pathology has been described as a “Dupuytren-like” contracture of the coracohumeral ligament and capsule which prevents external rotation14 and the tissue has been characterized by the presence of fibroblasts, proliferating fibroblasts, and chronic inflammatory cells.38
Although frozen shoulder is often considered to be self-limiting,36 full resolution of symptoms does not always occur,87 particularly in those who present with severe symptoms.39 Shaffer et al. monitored 68 patients over an average of 7 years, and found that half had mild pain or stiffness, while about one-third of the patients were objectively restricted when compared with the opposite unaffected side.87 This frequently protracted disease course has driven forward acceptable treatment modalities that will hasten recovery in terms of pain and function.
Secondary adhesive capsulitis develops when there is a known intrinsic (such as a rotator cuff or SLAP tear), extrinsic, or systemic cause.115 Possible causes of secondary frozen shoulder include microtrauma, macrotrauma, or postsurgical intervention, combined with prolonged immobilization of the shoulder. Most of the literature is directed toward outcomes following the treatment of primary adhesive capsulitis. Posttraumatic stiffness may result from soft-tissue contracture or bony malalignment from fracture, or a combination. The treatment of posttraumatic adhesive capsulitis may differ from that of primary adhesive capsulitis and thus prior to treatment, the cause of the stiffness should be determined.
EVALUATION
Codman22 described a set of diagnostic criteria that still holds true today, including pain in the shoulder which comes on slowly and is felt at the insertion of the deltoid, inability to
sleep on the affected side, atrophy of the scapular muscles, and local tenderness. In general, assessment should establish whether stiffness is idiopathic or secondary in nature, as well as the current stage of the condition, to direct appropriate treatment. History should focus on the level, nature, and timing (day or night) of the pain. Determine whether there are significant functional limitations such as the ability to dress and wash hair. Ask about previous injuries that may have resulted in bone or soft-tissue trauma. It is important to know if the patient has had treatment thus far, whether it is analgesics, physical therapy, injections, or previous surgery. Finally, inquire about medical conditions such as diabetes or thyroid disease which may predispose to idiopathic frozen shoulder.
sleep on the affected side, atrophy of the scapular muscles, and local tenderness. In general, assessment should establish whether stiffness is idiopathic or secondary in nature, as well as the current stage of the condition, to direct appropriate treatment. History should focus on the level, nature, and timing (day or night) of the pain. Determine whether there are significant functional limitations such as the ability to dress and wash hair. Ask about previous injuries that may have resulted in bone or soft-tissue trauma. It is important to know if the patient has had treatment thus far, whether it is analgesics, physical therapy, injections, or previous surgery. Finally, inquire about medical conditions such as diabetes or thyroid disease which may predispose to idiopathic frozen shoulder.
A comprehensive physical examination of the shoulder and cervical spine needs to be performed. Examination begins with inspection looking for atrophy of the deltoid, rotator cuff, and peri-scapular muscles. Palpation may reveal deep tenderness in the glenohumeral joint. Carbone et al.16 found that digital pressure over the coracoid area will elicit pain in the vast majority of patients (96%) with adhesive capsulitis compared to 2% of normal control subjects. They concluded that it can be considered an easy and reliable clinical test for identifying patients with or without this condition. The presence of pain with passive external rotation with the upper arm in a neutral position at the patient’s side and the elbow in 90 degrees of flexion has also been described as a useful test for adhesive capsulitis.108 The restrictions of shoulder motion are global in primary adhesive capsulitis. There is no consensus on the exact range of motion restriction required for a patient to be diagnosed as having a frozen shoulder and therefore the affected side should always be compared with the non-affected side. The examiner should evaluate passive motion and active motion in forward elevation in the plane of the scapula, external rotation at the side, and internal rotation. Glenohumeral motion should be measured while stabilizing the scapula to evaluate glenohumeral versus scapulothoracic range of motion. Patterns of motion loss are very important to recognize to determine anatomic location contracture; loss of external rotation in adduction indicates contracture of the rotator interval, limited external rotation in abduction is associated with contracture in the anteroinferior capsule, and limitation of internal rotation in either adduction or abduction is associated with posterior capsular contracture. Finally, assess the strength of the rotator cuff and evaluate for impingement symptoms and acromioclavicular joint pain.
It is essential to obtain standard anteroposterior, axillary, and outlet radiographs in the assessment of any patient presenting with persisting loss of shoulder motion. This will enable the physician to exclude obvious joint-related causes of loss of motion such as calcific tendonitis, osteoarthritis, previous fracture, chronic posterior dislocation, or tumor.84 An assessment of the presence of a subacromial spur can also be made on an outlet view, although acromial morphology is not implicated as a cause of primary adhesive capsulitis.82 Radiographs may often reveal osteopenia, but should not show any other definitive pathology.59,72 Other imaging is not routinely necessary where the diagnosis of idiopathic adhesive capsulitis is suspected.61 However, it may be helpful to exclude other pathology that may generate loss of motion such as rotator cuff tears, labral lesions, or early degenerative joint disease. Characteristic MRI findings in primary frozen shoulder are thickening of the coracohumeral ligament and the joint capsule in the rotator cuff interval17,23 and axillary recess,51 as well as complete obliteration of the fat triangle between the CHL and the coracoid process.63
MANAGEMENT
Initial conservative management, involving treatments such as anti-inflammatory drugs, corticosteroid injections, and physical therapy, may be successful in up to 90% of patients.54 However, some authors have reported that as much as 50% of patients have long-term residual pain and motion loss.39,87 The interventions available for the remaining refractory cases include closed manipulation under anesthesia (MUA), arthroscopic capsular release, and open capsular release. Whilst an initial period of nonoperative treatment is universally accepted, there are no clear guidelines as to the timing of surgical intervention. Generally, patients who are regressing despite appropriate therapy are likely to require surgical intervention. Levine et al.54 found that patients are more likely to fail nonoperative treatment if they initially present with worse declines in range of motion, fail to progress within 4 months of nonoperative treatment, or experience declines in range of motion from initial presentation. Frozen shoulder in patients with diabetes is generally more persistent and less responsive to nonoperative measures and therefore a risk factor for failed nonoperative treatment.62,69
Closed MUA has been used extensively with satisfactory short- and long-term results.26,29,37,74 However, some authors have reported that MUA does not offer any advantage over simple treatments such as home exercise52 or intraarticular steroid injections.47 Contraindications to manipulation include significant osteopenia, recent surgical repair of soft tissues about the shoulder, or the presence of fractures or neurologic injury.88 Furthermore, complications related to this procedure have been well documented, including fracture to both humeral head and glenoid,60 brachial plexus injury,9 as well as intraarticular soft tissue injury.58 These factors as well as advances in arthroscopy have led to many surgeons adopting arthroscopic capsular release for the treatment of refractory shoulder stiffness.
Conti24 is credited with the first partial arthroscopic shoulder release using a trocar and forceps to release the rotator interval, followed by intraarticular steroid and gentle manipulation. Ogilvie-Harris and Wiley70 initially used arthroscopic distension to rupture the capsule and later on blunt instruments or progressive cutting of the anterior capsule to treat frozen shoulder. They reported satisfactory outcomes in most of the 81 patients on whom they performed this technique with less satisfactory results in patients who had diabetes. Since these early technical descriptions and reports, the advantages of arthroscopic capsular release compared to closed MUA and open capsular release have become apparent.93 The outcomes of arthroscopic capsular release are discussed in detail at the end of this chapter. Arthroscopy allows for a visual confirmation of the diagnosis and degree of capsular contracture as well as the ability to diagnose and treat concomitant intraarticular and subacromial disease that may be contributing to the primary cause of the problem. This is performed without having to detach and then repair the subscapularis tendon that may be necessary with an open release and therefore there is no need to limit the patient’s range of motion to protect against tendon rupture.
The procedure allows for the controlled release of the entire pathologic capsule, as well as capsular resection and synovectomy which, in theory, may denervate the pain fibers and minimize the chance of recurrence. Furthermore, it may be performed in conjunction with a manipulation, in which case decreased force and torque on the humerus is required. Finally, whilst there is currently no definite supporting evidence, it is hoped that patients achieve more significant and rapid improvements in motion and pain compared to manipulation and open release.
The procedure allows for the controlled release of the entire pathologic capsule, as well as capsular resection and synovectomy which, in theory, may denervate the pain fibers and minimize the chance of recurrence. Furthermore, it may be performed in conjunction with a manipulation, in which case decreased force and torque on the humerus is required. Finally, whilst there is currently no definite supporting evidence, it is hoped that patients achieve more significant and rapid improvements in motion and pain compared to manipulation and open release.
There are a few notable contraindications to arthroscopic release. First, the surgeon should have adequate training and experience with shoulder arthroscopy and arthroscopic anatomy. It is important to be aware that it will be more difficult to enter and subsequently navigate within the contracted joint. Second, it is imperative that the surgeon recognizes that arthroscopy involves release of the tight and thickened glenohumeral capsule and thus will only be effective when the source of stiffness is primarily intraarticular. Extraarticular pathology typically necessitates open surgical release which is dealt with in the subsequent chapter. Relative contraindications to arthroscopic capsular release include cases in which the bony anatomy of the shoulder is altered and limits motion such as in certain posttraumatic situations. Also, patients who have had prior open surgery in the inferior capsular area, will have postoperative scarring which may distort the location of the axillary nerve making arthroscopic release hazardous.
SURGICAL ANATOMY
Capsuloligamentous Complex
Normal shoulder range of motion is achieved through a smooth glenohumeral joint with a lax capsular lining, free gliding of the rotator cuff tendons within the subacromial and subdeltoid spaces, and normal scapulothoracic mechanics.34,80 Adhesions or contracture in any of these locations can contribute to shoulder stiffness and distorted mechanics85 and thus should be identified as they may need to be addressed separately. It is imperative to have a good understanding of the pertinent anatomy and biomechanics of the normal shoulder capsuloligamentous restraints to shoulder motion. This will facilitate an understanding of the anatomical cause of the direction of motion loss and allow the surgeon to direct releases where loss of motion may be greatest.
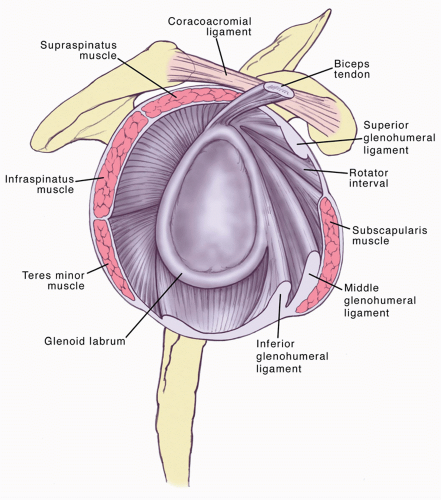
Glenohumeral joint stability is obtained by a combination of static and dynamic stabilizers (Fig. 10-1). The capsule
and associated ligaments are the most important static stabilizers.10 They help guide the positioning of the humeral head during normal shoulder movement, remaining lax throughout mid-range motion, whilst acting as a checkrein at end-range motion.94 Contracture of any given portion of this capsuloligamentous complex will cause premature reduction in glenohumeral motion as well as abnormal translation of the humeral head.40 Complete arthroscopic release of the glenohumeral joint aims to significantly increase glenohumeral range of motion and translation, whilst stability is maintained by the intact rotator cuff myotendinous units.65 The release should address three portions of the capsule, each requiring distinct release techniques, namely the rotator interval and anterior capsule, the inferior capsule, and the posterior capsule.
and associated ligaments are the most important static stabilizers.10 They help guide the positioning of the humeral head during normal shoulder movement, remaining lax throughout mid-range motion, whilst acting as a checkrein at end-range motion.94 Contracture of any given portion of this capsuloligamentous complex will cause premature reduction in glenohumeral motion as well as abnormal translation of the humeral head.40 Complete arthroscopic release of the glenohumeral joint aims to significantly increase glenohumeral range of motion and translation, whilst stability is maintained by the intact rotator cuff myotendinous units.65 The release should address three portions of the capsule, each requiring distinct release techniques, namely the rotator interval and anterior capsule, the inferior capsule, and the posterior capsule.
Rotator interval contracture of the coracohumeral ligament restricting external rotation is the characteristic pathology in frozen shoulder66,76 and therefore its release is a critical portion of the surgical procedure. The rotator interval is a triangular space located in the anterosuperior portion of the glenohumeral joint, bounded by the supraspinatus superiorly and the subscapularis inferiorly, and the coracoid process forms its medial base.31 Its contents include the coracohumeral ligament, the superior glenohumeral ligament, the glenohumeral capsule, and the biceps tendon. Anatomic studies have demonstrated that the rotator interval functions as a restraint to excessive motion, provides inferior glenohumeral stability in the adducted position,41 and gives stability to the long head of the biceps.2 The rotator interval is maximally tensioned with the arm adducted in external rotation. Several authors have emphasized the importance of releasing the rotator interval capsule and, more importantly, the coracohumeral ligament, in addition to other portions of the capsule. Tetro et al.95 performed a cadaveric study to assess the feasibility of completely releasing the rotator interval, capsule, and coracohumeral ligament arthroscopically. Their technique involved beginning the release just superior to the superior border of the subscapularis, then proceeding superiorly until the anterior edge of the biceps tendon is reached. The dissection is performed from deep to superficial, cutting through the capsule first and the resection is then performed until the coracoacromial ligament is visualized. Despite the fact that the coracohumeral ligament is an extraarticular structure, the authors were able to reproducibly section the ligament using this technique.
The middle glenohumeral ligament restricts motion with the arm in external rotation in the midrange of abduction30; this structure is identified deep to the subscapularis and is divided as part of the anterior release. The anterior and posterior inferior glenohumeral ligaments form a sling beneath the glenohumeral joint that stabilizes the joint in positions of full abduction, preventing anterior, posterior, and inferior translation.75,98 The posterior capsule, the thinnest part of the capsule, lies proximal to the superior portion of the posterior band of the inferior glenohumeral ligament. The most superior portion of the posterior capsule becomes tensioned with the arm adducted and internally rotated, whereas tension shifts to the inferior portion with abduction and internal rotation.15 Release of the posterior capsule is necessary to treat any deficit in internal rotation.110
Axillary Nerve
By virtue of its relation to the inferior joint capsule, the axillary nerve assumes importance in shoulder arthroscopy, particularly in the setting of a joint contracture where it may be tethered to tissue. Early technical descriptions of arthroscopic release suggested avoiding inferior instrumented release to prevent axillary nerve damage.79 Knowledge of the anatomy of the axillary nerve in relation to the glenoid rim and the capsule aids the shoulder surgeon in avoiding iatrogenic nerve injury during arthroscopic capsular release. Several authors have investigated the anatomy of the axillary nerve with particular reference to arthroscopic procedures.12,27,48,81,101,113 Uno et al.101 described the arthroscopic location of the axillary nerve via an extraarticular dissection, using needles to mark it, and then observing the position of the needles and the relation to the inferior glenoid rim through an arthroscopic view. They found that the axillary nerve was adherent to the shoulder capsule with loose areolar tissue in the zone between 5 and 7 o’clock and was closest to the glenoid in the neutral position, in extension, and in internal rotation. With shoulder abduction, external rotation, and perpendicular traction, the capsule became taut and the axillary nerve moved away from the glenoid. They concluded that abduction, external rotation, and perpendicular traction would increase the zone of safety during arthroscopic anteroinferior capsulotomy adjacent to the glenoid between the 5 and 7 o’clock positions. Jerosch et al.48 also determined the distance between the capsule and the axillary nerve in different joint positions. The shortest distance between the insertion of the inferior capsule and the axillary nerve was measured at the glenoid and humeral insertions in abduction, adduction, internal, and external rotation. They also found that the axillary is closer to the humeral than to the glenoid attachment of the joint capsule and that the distance to the nerve is greatest in abduction and external rotation. Price et al.81 provided an understanding of the branching pattern of the axillary nerve and its relation to the IGHL and glenoid rim. In their cadaveric dissections, the teres minor branch was the closest to the glenoid rim. They also performed coronal sectioning of shoulder specimens, which demonstrated that the closest point between the axillary nerve and the glenoid rim was at the 6 o’clock position on the inferior glenoid rim. At this position, the average distance between the axillary nerve and the glenoid rim was 12.4 mm. The axillary nerve lay, throughout its course, at an average of 2.5 mm from the inferior glenohumeral ligament. Zanotti and Kuhn,113 in a cadaveric study, performed an arthroscopic circumferential capsular release using an electrocautery tip approximately 1 cm lateral to the glenoid rim while the arm was in a position of 45 degrees abduction and 20 degrees flexion. Subsequent dissection showed that in all specimens the axillary nerve lay anterior to the inferior edge of the subscapularis muscle at the level of the glenoid rim and it continued laterally and inferiorly under the subscapularis close to the inferior capsule approximately 17 mm lateral to the inferior glenoid rim. These findings indicated that a capsular incision a few millimeters lateral to the glenoid labrum should not place the nerve at risk for injury. Finally, Yoo et al.112 examined the morphologic features of the axillary nerve and its relation to the glenoid under an arthroscopic setup with different arm positions. They found that the closest points from the glenoid were between the 5:30 and 6:00 o’clock positions and the closest distance range varied from 10 to 25 mm in the neutral arm position. The abductionneutral position resulted in the greatest distance between the inferior glenoid and the nerve.
Procedure
It is preferable to perform this procedure under inter-scalene block, in addition to general anesthesia, so that passive range of motion therapy can be started immediately after surgery. The patient can be positioned in either beach chair or lateral depending on the surgeon’s preference. An arthroscopic pump is required to control intraarticular fluid pressure; pressure is generally kept to a minimum to reduce swelling but can be temporarily increased if bleeding is encountered. Epinephrine in the irrigating fluid and hypotensive anesthesia are helpful ways of further improving visualization. A 90 degree angled or hooked tip radiofrequency device is needed to allow for controlled division of tissue whilst minimizing bleeding. An arthroscopic capsular punch is typically used to divide the inferior capsule and it is useful to have a shaver to remove synovitis and debride residual capsule (Fig. 10-2).
Examine and document the range of shoulder motion in both shoulders prior to draping the patient. Some surgeons prefer to perform an initial manipulation of the shoulder prior to introducing the arthroscope. The potential advantage of this is to allow for easier and safer insertion of the arthroscope.100 However, manipulation will cause significant bleeding (Fig. 10-3) making visualization difficult for the remainder of the procedure, and capsular rupture allows for fluid extravasation with resulting soft-tissue distension. Furthermore, manipulation may result in iatrogenic injury to intraarticular structures including labral and rotator cuff tears.58
After skin preparation and draping, a standard posterior viewing portal is made. Palpate the humeral head and glenoid with the introducer to confirm correct location and pass the arthroscope into the joint. It is more difficult than usual to enter the joint due to the thickened posterior capsule and the diminished intracapsular space. Great care should be taken not to damage the articular cartilage surface; it may be helpful to aim at the tip of the arthroscope toward the superior aspect of the glenoid where the available joint space is larger. Blunt dissection with a hemostat to identify the articular margins as well as distraction of the glenohumeral joint are other useful measures if it remains difficult to insert the arthroscope.
The rotator interval will be seen to be contracted, thickened, and inflamed with synovitis (Fig. 10-4). Perform a diagnostic arthroscopy and evaluate the joint fully for other associated pathology such as rotator cuff disease, biceps pathology, or degenerative changes around the articular surface. An anterior portal is now made using an outside to inside technique with an 18-gauge spinal needle in the superolateral portion of the rotator interval just beneath the biceps tendon. An anterior cannula is then inserted to allow for outflow to control swelling and prevent heating of fluid that can potentially lead to cartilage damage114 and subsequent chondrolysis.49 Use the radiofrequency device to cauterize any hypervascular synovitis and ablate the rotator interval tissue (Fig. 10-5). This will significantly open the joint and provide an increase in external rotation, allowing easier navigation around the joint. With progressive rotator interval release, identify the coracoid and divide the coracohumeral ligament. The conjoined tendon can be seen inferior to the coracoid and should be protected. As the release continues through the full depth of the tissue, the vertical fibers of the coracoacromial ligament, which is the
most superficial structure, become apparent. This is an important landmark as it confirms full thickness release of the interval.95 The coracoacromial ligament is not directly involved in the disease process and need not be divided. To complete the lateral and superior interval release, work alongside the biceps tendon. Care should be taken at the lateral extent of the release to protect the stabilizing sling of the long head of the biceps.89 Once the interval has been released, the middle glenohumeral ligament and the remaining anterior capsule should be divided. This relies on identifying the plane between the upper border of the subscapularis, which should be protected, and the deeper capsular layer. The release is continued inferiorly, staying about 5 mm to the labrum, down to the level of the inferior glenohumeral ligament (approximately the 5 o’clock position in a right shoulder) under direct vision.
most superficial structure, become apparent. This is an important landmark as it confirms full thickness release of the interval.95 The coracoacromial ligament is not directly involved in the disease process and need not be divided. To complete the lateral and superior interval release, work alongside the biceps tendon. Care should be taken at the lateral extent of the release to protect the stabilizing sling of the long head of the biceps.89 Once the interval has been released, the middle glenohumeral ligament and the remaining anterior capsule should be divided. This relies on identifying the plane between the upper border of the subscapularis, which should be protected, and the deeper capsular layer. The release is continued inferiorly, staying about 5 mm to the labrum, down to the level of the inferior glenohumeral ligament (approximately the 5 o’clock position in a right shoulder) under direct vision.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree