Anteroinferior Capsular Shift for Involuntary Multidirectional Instability
Robert M. Orfaly
Michael A. Wirth
Charles A. Rockwood Jr
Multidirectional instability (MDI) is a common condition but is often misdiagnosed, poorly understood, and inappropriately treated. As the joint with the greatest arc of motion in the body, bony stability is inherently poor, and there is significant laxity in the normal shoulder. This laxity is normally controlled by several mechanisms including the adhesion/cohesion effect of the synovial fluid, the concavity produced by the bony glenoid and increased by the glenoid cartilage and labrum, and the static restraint of the glenohumeral ligaments at the extremes of motion. Laxity is also controlled by the dynamic contraction of the rotator cuff muscles to directly counterbalance a dislocating force and to create a joint reaction force that serves to center the convex humeral head in the concave glenoid.
Instability can therefore be defined as the aberration or failure of any combination of the above stabilizing mechanisms to maintain physiological laxity and leading to symptomatic joint subluxation or dislocation. Generalized ligamentous laxity can contribute to MDI of the shoulder. However, not all cases of MDI are associated with generalized laxity (1). At the same time, not all cases of hyperlaxity are symptomatic. Even within the same glenohumeral joint, there may be unidirectional instability but multidirectional laxity. Jia et al. (1) found that the ability to sublux the humeral head over the glenoid rim during examination under anesthesia is common regardless of the operative diagnosis. However, higher grades of laxity were associated with instability in all directions. Aberrations in joint proprioception may also play a role in MDI (2).
One of the most widely utilized means of categorizing glenohumeral instability is by its directionality. There are indeed important differences in presentation and treatment between the classic traumatic recurrent anterior shoulder instability and atraumatic MDI. However, these conditions should most accurately be seen as existing on a spectrum. As noted above, unidirectional dislocations can be associated with multidirectional laxity. MDI does not always present atraumatically in the lax female patient. In one series, 61% of MDI patients were male, 72% were defined as athletic, and 50% presented with a traumatic history of onset (3). This series also found 24% of patients at surgery to have a capsular Bankart lesion or glenoid fracture. Another study found that nearly half the patients enrolled with MDI had concomitant pathology (4). Three-quarters of these had Bankart lesions.
INDICATIONS/CONTRAINDICATIONS
Since the classic description of this condition by Neer and Foster (5), a higher index of suspicion and a better understanding of MDI have led to increased recognition of this condition and improved surgical outcomes. Most patients with MDI will respond favorably to a rigorous, physician-directed, home exercise program (6).
Involuntary multidirectional shoulder instability rarely requires surgical intervention, but when surgery is necessary, methods of operative intervention that provide satisfactory results for traumatic instability have not uniformly worked for patients with MDI. In these patients, an anteroinferior capsular shift, designed to tighten the capsule, decreasing its overall volume, has proven successful when a properly directed rehabilitation program fails to stabilize the shoulder (5).
Involuntary multidirectional shoulder instability rarely requires surgical intervention, but when surgery is necessary, methods of operative intervention that provide satisfactory results for traumatic instability have not uniformly worked for patients with MDI. In these patients, an anteroinferior capsular shift, designed to tighten the capsule, decreasing its overall volume, has proven successful when a properly directed rehabilitation program fails to stabilize the shoulder (5).
The primary indication for operative treatment of MDI is symptomatic, involuntary, global glenohumeral instability in a patient who has no psychological impairment and has failed a course of at least 6 months of conservative treatment in a physician-directed therapy program aimed at strengthening the rotator cuff, deltoid, and scapular stabilizers. The two essential components of the reconstruction involve closure of the rotator interval capsule and reduction of the excessive joint volume through selective and anatomic plication of the redundant anterior, inferior, and posteroinferior aspects of the capsule without creating significant limitations in external rotation. Attention to these issues is important as both instability symptom resolution and motion preservation have been shown to influence patient satisfaction (2). Patients with traumatic initial episodes are less likely to respond to a conservative treatment program and are more likely to have a Bankart-Perthes lesion noted on preoperative imaging or at surgery.
Contraindications to this procedure relate primarily to patient selection. Absolute contraindications include documented emotional or psychological disturbances affecting rehabilitation potential, glenoid aplasia or hypoplasia (7), and noncompliance with previous preoperative therapy regimen. Relative contraindications include motivation by secondary gain (e.g., some workman’s compensation cases) and significant neurologic injury to the axillary or suprascapular nerves. Although a history of multiple prior surgical procedures on the joint does decrease the likelihood of a good result, it is not a contraindication to performing an anteroinferior capsular shift. Patients with known collagen abnormalities, such as Ehlers-Danlos syndrome, may well respond to surgical stabilizations; however, the predictability of complete resolution of symptoms is not always present. We have obtained good results in most revision cases using this technique, although extra attention to details is of paramount importance (8, 9).
Patients who have involuntary MDI and have completed at least a 6-month course of conservative management with no improvement are candidates for anteroinferior capsular shift, although we may continue patients with atraumatic instability in their rehabilitation program for a full year before opting for surgical intervention. A major benefit of a lengthy preoperative conservative treatment program is the opportunity to evaluate the patient’s character, which plays a significant role in the eventual outcome of any management strategy. We stress to patients that a significant portion of the success or failure of their treatment is dependent upon their willingness to participate in and direct their preoperative and postoperative rehabilitation. This gives the patients a sense of control over their outcome and encourages an active role in their treatment. At the same time, at least one study has suggested that young, athletic patients may have a relatively poor response to nonoperative treatment (10). Capsular shift appears to be equally successful in patients with unilateral MDI who participate in contact sports (11).
PREOPERATIVE PLANNING
Together, the history, physical examination, and radiographic examinations create a picture of the unstable shoulder that allows accurate preoperative planning. In contemplating surgical treatment for MDI of the shoulder, it is essential to determine whether the primary direction of instability is anterior, inferior, or posterior. This particular information is generally gleaned from the history given by the patient. Patients with predominantly anterior instability will describe symptoms that occur when their arm is in the apprehension position of abduction and external rotation, such as when they cock their arm to throw a ball. Patients with predominantly posterior instability will complain that their shoulder tends to “slip out of place” when their arm is forward flexed and internally rotated, such as when they remove a book from an overhead shelf. Inferior instability will most often be demonstrated when patients carry objects at their side, such as a heavy suitcase.
Patients with MDI often give a history of shoulder complaints beginning in childhood and initial episodes of subluxation or dislocation that were atraumatic or occurred with minimal trauma. However, as noted above, patients can present with histories across a spectrum of atraumatic or traumatic onset. Involuntary, symptomatic instability results gradually from multiple recurrences or begins after a traumatic event. Dislocations in patients with MDI are frequently transient and often do not require the assistance of a physician or another person to obtain a reduction. Painful subluxation is generally associated with global instability secondary to traumatic events rather than with MDI of an entirely atraumatic origin.
Physical examination of the patient with MDI usually reveals generalized ligamentous laxity as evidenced by hyperextension of the elbows, knees, and metacarpophalangeal joints; hyperabduction of the thumb (passive abduction of the thumb to the forearm with the wrist flexed); and patellofemoral laxity. Many patients with MDI can voluntarily sublux their shoulders, usually in a posterior direction. This ability does not absolutely contraindicate surgical intervention, but patients with voluntary subluxation should undergo psychiatric evaluation. Instability testing, by definition, shows laxity in both the anteroposterior (AP) and inferior directions, manifested by a positive sulcus sign (a dimple created between the humeral head and the acromion when the humeral shaft is pulled distally), a positive anterior or posterior drawer (load and shift)
test (anterior or posterior subluxation noted when directly pushing the humeral head anteriorly or posteriorly, after centering the head in the glenoid fossa), and a positive push-pull test (more than 50% posterior translation of the shoulder in the supine patient when the wrist is pulled up, while the proximal humerus is pushed downward). Rotator cuff and deltoid strength are typically normal, although patients with MDI can often differentially contract the heads of the deltoid, causing subluxation. Even if strength is normal, coordination of muscular contractions may be pathological. Asynchrony of scapulothoracic motion can often be noted on physical examination and has been documented in this population with kinematic testing (12). Therapy aimed at improving strength, coordination, and endurance, particularly in the rotator cuff and scapular stabilizers, helps to control dynamic instability by improving scapular positioning and increasing and centering the joint reaction force in the glenoid, thus improving stability through increased concavity compression.
test (anterior or posterior subluxation noted when directly pushing the humeral head anteriorly or posteriorly, after centering the head in the glenoid fossa), and a positive push-pull test (more than 50% posterior translation of the shoulder in the supine patient when the wrist is pulled up, while the proximal humerus is pushed downward). Rotator cuff and deltoid strength are typically normal, although patients with MDI can often differentially contract the heads of the deltoid, causing subluxation. Even if strength is normal, coordination of muscular contractions may be pathological. Asynchrony of scapulothoracic motion can often be noted on physical examination and has been documented in this population with kinematic testing (12). Therapy aimed at improving strength, coordination, and endurance, particularly in the rotator cuff and scapular stabilizers, helps to control dynamic instability by improving scapular positioning and increasing and centering the joint reaction force in the glenoid, thus improving stability through increased concavity compression.
Although patients with MDI often have normal radiographic findings, an instability series consisting of AP views in internal and external rotation, an axillary lateral view, a Stryker notch view, and an apical oblique view should be obtained. These patients can have traumatic episodes superimposed on a background of generalized ligamentous laxity, and the presence of a Bankart-Perthes lesion has implications for the outcome of a conservative treatment program. It is also important to recognize the presence of these lesions preoperatively, as all Bankart-Perthes lesions and extremely severe Hill-Sachs lesions should be addressed at the time of surgery. The axillary lateral view should be scrutinized for evidence of glenoid hypoplasia or aplasia or excessive glenoid retroversion, as these conditions are poorly treated by anterior capsular reconstructions alone. Patients with glenoid retroversion abnormalities should undergo computed tomography scanning of both shoulders including the scapular bodies to further delineate version more precisely.
An examination under anesthesia is not necessary to make the diagnosis of MDI, but it can be performed in the operating room before anteroinferior capsular shift.
Although we recognize the growing enthusiasm of the orthopaedic community for arthroscopic instability repair, we continue to believe that the best way to address MDI is with an open procedure. Excessive capsular laxity is the primary pathology in MDI, and it is difficult to achieve similar volume reductions and to check the degree of tightening achieved intraoperatively when performing a reconstruction arthroscopically (13). Thermal capsular shrinkage with radiofrequency probes or laser has been demonstrated to produce unacceptably high failure rates and their use has largely been abandoned (4, 14, 15, 16). Open surgery allows for capsular sectioning and repair that allows intraoperative assessment of the amount of capsular redundancy and adjustment of the capsular tension according to the patient’s individual needs (3, 9). In addition, anterior labral pathology, if present, is easily addressed at the time of open capsular shift. Although the history, physical examination, and radiographic studies for MDI are generally clear, in rare situations, when the diagnosis remains obscure, diagnostic arthroscopy may help clarify treatment decisions. Posterior and superior labral pathologies are also being recognized with increasing frequency and, if present, may be addressed arthroscopically before performing an open capsular shift.
SURGERY
The anteroinferior capsular shift procedure described below was developed and used by the senior author (CAR) beginning in 1970 and was first described in 1984 (17). The technique incorporates aspects of the methods of Bankart and Putti-Platt, as well as the classic inferior capsular shift described by Neer and Foster (5) in 1980. In addition, there are several important alterations in this procedure:
Only the upper two-thirds of the subscapularis tendon is reflected from the capsule; the lower one-third of the muscle-tendon unit is left intact, which may spare some proprioceptive capacity, in addition to preserving the primary blood supply to the humeral head, the anterior humeral circumflex vessels.
Elevation and retraction of the lower one-third of the subscapularis tendon from the capsule provides protection of the axillary nerve while incising and shifting the inferior capsule.
The capsule is freed from the overlying subscapularis tendon from top to bottom.
The capsular incision is made halfway between the glenoid rim and the attachment of the capsule to the humeral neck and extends from the rotator interval down to, and if necessary, beyond the 6 o’clock position; division of the capsule in this fashion allows the thin anterior capsule to be strengthened by double-breasting it in the midportion rather than shifting it medially on the glenoid or laterally on the humerus.
Rather than shifting the capsule with the arm held in one position, a “selective capsular shift” may be performed. This entails placing the initial inferior suture and checking shoulder external rotation with the arm in a throwing position. The next sutures are placed and the external rotation checked in midrange. Finally, the sutures in the superior capsule and rotator interval are placed and the external rotation is checked with the arm at the side. This provides a very anatomic reconstruction with no restriction of external rotation throughout the entire range of motion.
When the shift is completed, the detached medial upper two-thirds of the subscapularis tendon is repaired to its lateral stump in an end-to-end fashion without shortening, minimizing the risk of internal rotation contracture.
We have found that these simple modifications of the procedure provide us with satisfactory, predictable, and reproducible results.
Preparing the Patient
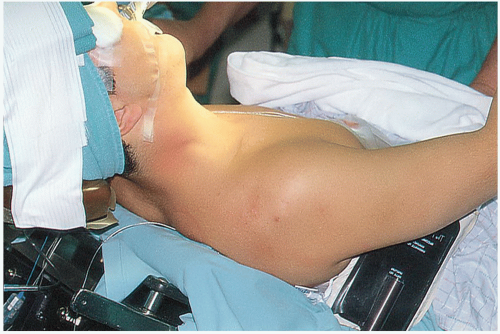
We prefer to perform the procedure under general anesthesia with the patient in a semi-Fowler or beach-chair position. Additional use of interscalene blocks for regional anesthesia can be of significant benefit for postoperative pain control. We remove the headrest from the standard operative table and replace it with a special headrest. In the past, we have used a Mayfield headrest (Ohio Medical Instrument Co., Inc., Cincinnati, OH), which can be commonly found in most operating rooms. Currently, there are several operating table attachments designed specifically to provide better access to the shoulder for the assistant at the head of the table and allow the patient to be moved toward the edge of the table (Fig. 21-1). With all of these attachments, the patient’s head must be adequately secured to prevent dangerous sliding as the arm is moved during the case. A bolster or kidney rest to stabilize the torso is also valuable. It is important to evaluate an attachment for the presence of padding secured to the forehead to prevent injury by the first assistant’s elbow.
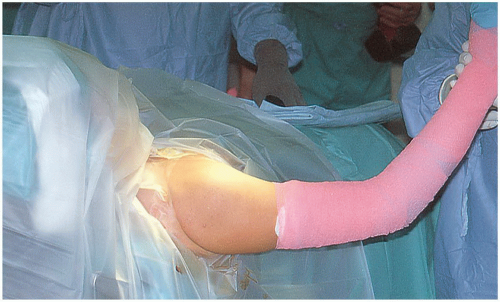
The shoulder is prepped and the arm draped free (Fig. 21-2), affording the primary surgeon a comfortable position in the axilla to perform the procedure. It is critical that the surgeon has an adequate number of assistants to perform the procedure properly. A mechanical arm holder that can assist in positioning the extremity or a second skilled assistant is generally required in order to provide appropriate access and exposure for the surgeon. In general, antibiotic prophylaxis is given either as a single dose or for 24 hours (18). With the increased recognition of fastidious organisms such as Propionibacterium acnes as an important pathogen in shoulder infections, preoperative workup and antibiotic selection should be carefully considered in revision cases.
The Incision and Approach
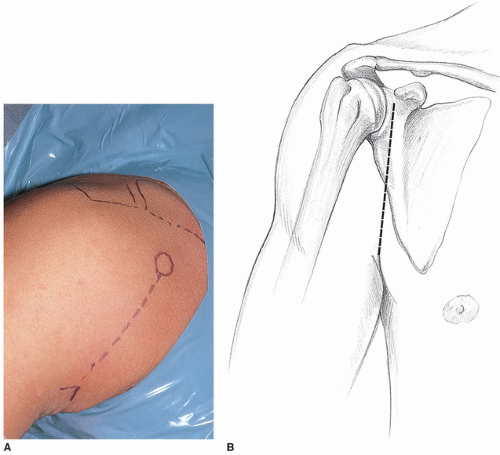
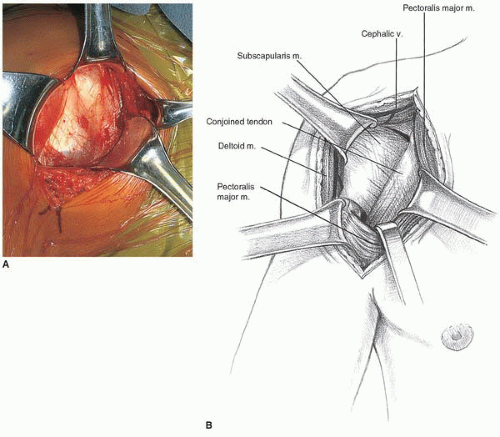
The standard axillary incision extending from the coracoid process into the axilla following the anterior axillary fold is used for the approach (Fig. 21-3). If necessary, this incision can be extended proximally toward the clavicle in large, muscular males. For a more cosmetic scar in females, the incision can be modified in the manner described by Leslie and Ryan (19), with the incision in the axilla. This pure axillary incision necessitates more extensive proximal subcutaneous dissection to the level of the clavicle for exposure of the deltopectoral interval. The incision through the dermis is performed with a no. 10 blade, and then Bovie electrocautery is used in the cutting mode to complete the incision down through the subcutaneous tissue. Curved Mayo scissors are used to undermine the subcutaneous tissue superomedially and inferolaterally (Fig. 21-4). This effectively exposes the deltopectoral interval, which crosses the incision diagonally and can be identified by the presence of the cephalic vein (Fig. 21-5). Occasionally, the cephalic vein is not readily identifiable, as it may be absent or deep within the deltopectoral interval. When this is the case, the interval is more easily identified proximally where there is greater divergence of the muscle fibers of the deltoid and pectoralis major, which are also separated by the easily palpated coracoid process. The cephalic vein is carefully preserved and taken laterally with the deltoid, preserving all feeders from the deltoid. Because we have seen increased venous congestion and postoperative discomfort in patients with cephalic vein disruption, we do not recommend ligation of the cephalic vein and will even repair small inadvertent perforations in the vein with interrupted 8-0 nylon sutures. Blunt fingertip dissection can then define the deltopectoral interval from the clavicle down to the insertion of the pectoralis major tendon just lateral to the bicipital groove. There is no need to detach any portion of the deltoid from its origin on the clavicle or its insertion on the humerus. Releasing the proximal 1 to 2 cm of the pectoralis major insertion can be helpful for improving visualization of the inferior capsule, but care must be taken not to injure the tendon of the long head of the biceps, which lies in the bicipital groove just medial and deep to the pectoralis major insertion. We do not recommend detachment of the coracoid process or the conjoined tendon. Narrow Richardson retractors are used to hold the deltoid laterally and the pectoralis major medially. The clavipectoral fascia overlies the subscapularis and the conjoined tendon and is continuous with the coracoacromial ligament proximally (Fig. 21-6). This fascia is divided vertically just lateral to the muscle belly of the short head of the biceps, which generally projects laterally from beneath the conjoined tendon. The coracoacromial ligament is recognized as an important anterosuperior restraint for the humeral head and should be preserved. The conjoined tendon is freed from its investing fascia, and the clavipectoral fascia is released proximally to the level of the coracoacromial ligament attachment to the lateral aspect of the coracoid.
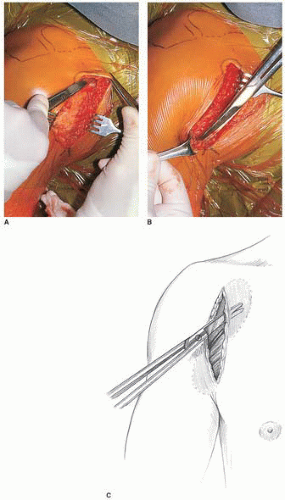
 FIGURE 21-5 A: The cephalic vein demarcates the deltopectoral interval. B: The cephalic vein is retracted laterally with the deltoid. |
The musculocutaneous and axillary nerves are then located, palpated, and protected. The entry point of the musculocutaneous nerve into the conjoined tendon complex is quite variable, though ordinarily it is approximately 5 cm distal to the tip of the coracoid (20). Digital palpation just deep and medial to the conjoined tendon and muscles will reveal the region of penetration of the musculocutaneous nerve (Fig. 21-7). The axillary nerve is at higher risk during the inferior capsular shift procedure (Fig. 21-8) and can be palpated by sliding the index finger down along the anterior border of the subscapularis until it reaches the 6 o’clock position, where the axillary nerve can be hooked with the index finger as it dives posteriorly and lies inferior to the capsule as it approaches the quadrangular space (Figs. 21-9 and 21-10). A large Richardson or a self-retaining Kolbel retractor is placed beneath the conjoined tendon to expose the underlying subscapularis muscle and tendon (Fig. 21-11A). It is extremely important that retraction on the conjoined tendon not be overly vigorous, as a transient musculocutaneous palsy may result. In the same manner, a blunt retractor such as a Scoffield can be used to add protection for the axillary nerve, but vigorous retraction must be avoided.
Technique
After sweeping the remaining fascia from the surface of the subscapularis and irrigating the wound, the upper and lower borders of the subscapularis tendon are identified. The “soft spot” of the rotator interval (i.e., the natural demarcation between the tendons of the supraspinatus and subscapularis) marks the superior border of the subscapularis tendon, and the “three sisters” (i.e., the anterior humeral circumflex artery and its two accompanying veins) can routinely be identified just proximal to the inferior border of the subscapularis tendon (Fig. 21-11B). This also correlates with the region of the lower one-third of the subscapularis that remains muscular nearly to its humeral insertion. The rotator interval is often patent in patients with MDI. If not, a small Darrach retractor is placed in the rotator interval to help define the superior border of the subscapularis tendon, and if necessary, a ball-tipped pusher can be used to push the humeral head posteriorly to enhance exposure. With the arm externally rotated to expose the entire tendon (Fig. 21-11B), the upper two-thirds of the subscapularis tendon is vertically transected with electrocautery approximately 1.5 to 2 cm medial to its insertion on the lesser tuberosity (Figs. 21-12, 21-13 and 21-14). Care is taken to avoid penetration of the underlying anterior capsule while separating the subscapularis tendon from the adherent capsule. The tendon is least adherent to the capsule medially and inferiorly. A Cobb elevator can sometimes be used to good effect to dissect the interval in this region. The separation can then be sharply dissected from medial to lateral. A small portion of the subscapularis tendon may be left attached to the capsule, as this may supply some extra strength to the capsular repair. The inferior one-third of the subscapularis tendon is not transected. This maintains the integrity and some proprioception of the muscle-tendon unit and preserves the anterior humeral circumflex vessels, which provide the vast majority of the blood supply to the humeral head. Once the vertical transection is completed, electrocautery is used to create a horizontal incision in line with the fibers of the subscapularis tendon and muscle between the upper two-thirds and lower one-third of the subscapularis tendon. Mayo scissors are used to cautiously reflect the upper two-thirds of the subscapularis from the underlying capsule, until that portion of the subscapularis muscle and tendon is a free, dynamic unit (Fig. 21-15). Again, it is important to avoid injury to the underlying capsule. Several nonabsorbable no. 1 cottony Dacron sutures are placed as stay sutures in the lateral edge of the medial portion of the subscapularis tendon for use in retraction and later repair (Fig. 21-16). One-millimeter cottony Dacron tapes can be used in the subscapularis if the patient is large, strong, and extremely active. A no. 15 blade is used to free the lateral stump of the subscapularis from the capsule to facilitate the final tendon repair (Figs. 21-17 and 21-18). A periosteal elevator is used to gently strip the intact inferior portion of the subscapularis from the anteroinferior capsule until the entire capsule can be visualized from the 12 to the 6 o’clock positions (Fig. 21-19). A Scoffield retractor can then be placed inside the lower third of the subscapularis to expose the inferior capsule and protect the axillary nerve during the capsular shift (Figs. 21-20 and 21-21). This is followed by copious wound irrigation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree