Abstract
Being able to predict an individual’s potential for recovery of motor function after stroke may facilitate the use of more effective targeted rehabilitation strategies, and management of patient expectations and goals. This review summarises developments since 2010 of approaches based on clinical, neurophysiological and neuroimaging measures for predicting individual patients’ potential for upper limb recovery. Clinical assessments alone have low prognostic accuracy. Transcranial magnetic stimulation can be used to assess the functional integrity of the corticomotor pathway, and has some predictive value but is not superior when used in isolation due to its low negative predictive value. Neuroimaging measures can be used to assess the structural integrity of descending white matter tracts. Recent studies indicate that the integrity of corticospinal and alternate motor tracts in both hemispheres may be useful predictors of motor recovery after stroke. The PREP algorithm is currently the only sequential algorithm that combines clinical, neurophysiological and neuroimaging measures at the sub-acute stage to predict the potential for subsequent recovery of upper limb function. Future research could determine if a similar algorithmic approach may be useful for predicting the recovery of gait after stroke.
Résumé
Prédire la capacité individuelle du patient à récupérer ses fonctions motrices après AVC peut faciliter une utilisation plus efficace de stratégies ciblées de rééducation fonctionnelle, et une meilleure prise en charge des attentes et objectifs des patients. Cette revue résume depuis 2010 les développements des approches prédictives du potentiel individuel de récupération motrice du membre supérieur de chaque patient basées sur des mesures cliniques, neurophysiologiques et d’imagerie. Les évaluations cliniques seules ont une valeur prédictive faible. La stimulation magnétique transcranienne peut être utilisée dans l’évaluation de l’intégrité fonctionnelle de la voie corticomotrice, et montre une certaine valeur prédictive mais n’est pas supérieure quand elle est utilisée seule à cause de sa faible valeur prédictive négative. Les mesures de neuroimagerie peuvent évaluer l’intégrité structurelle des faisceaux descendants de la substance blanche. De récentes études montrent que l’intégrité du faisceau corticospinal et des voies motrices indirectes au sein des deux hémisphères peuvent se révéler des marqueurs prédictifs utiles de la récupération motrice post-AVC. L’algorithme PREP est actuellement le seul algorithme séquentiel combinant des mesures cliniques, neurophysiologiques et de neuroimagerie durantla phase subaiguë de l’AVC, qui est capable de prédire le potentiel de récupération motrice du membre supérieur. De futures études pourraient déterminer si une approche algorithmique similaire pourrait se révéler utile pour prédire la récupération de la marche post-AVC.
1
English version
1.1
Introduction
Stroke is the third most common cause of long-term adult disability in developed countries . There are nearly 800,000 strokes a year in the United States alone, with a third of all stroke survivors experiencing varying levels of disability . The ability of stroke survivors to independently undertake activities of daily living is dependent on the recovery of motor function, particularly of the upper limb .
Recovery of motor function is characterized by the individual’s ability to perform movements using the same effectors and muscle activation patterns in the same manner as prior to stroke . Recovery is highly variable within the initial days after stroke when rehabilitation begins, making it difficult to estimate with any great degree of accuracy the extent of motor recovery that will be obtained months after stroke at the end of rehabilitation. The ability to predict an individual’s potential for motor recovery could add value because it would allow for individually-tailored rehabilitation, management of patient and therapist expectations, and may result in more effective utilization of health resources.
This review provides an update on developments since 2010, when we proposed an algorithm for predicting the potential for recovery of upper limb function for individual patients . Here we identify new studies on the key predictors of motor recovery post-stroke that provide general support for the proposed algorithm, and extend the idea to the lower limb.
1.2
A multimodal approach
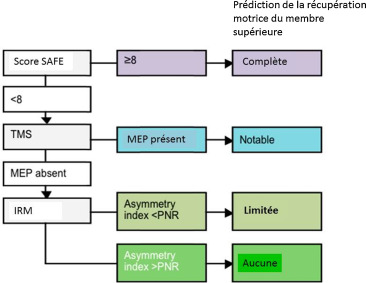
Currently, three main methods have been identified for evaluating capacity for motor recovery in the initial days after stroke: clinical assessment scales, neurophysiological assessments, and neuroimaging techniques. In 2010 we proposed an algorithm to sequentially combine these measures to make accurate prognoses of upper limb recovery for individual patients as they begin rehabilitation, at the sub-acute stage of stroke. The algorithm begins with a clinical assessment within 72 hours of symptom onset . Shoulder abduction and finger extension strength are each graded out of 5 using the Medical Research Council (MRC) grades, and then summed to produce a SAFE score (Shoulder Abduction, Finger Extension), with a maximum score of 10. This simple bedside test can identify patients with excellent potential for motor recovery in the upper limb. Patients with a SAFE score of 8 or more have the potential to make a complete or near-complete recovery within 12 weeks.
Patients with a SAFE score below 8 then have a neurophysiological assessment within a week after symptom onset. Transcranial magnetic stimulation (TMS) is used to assess the functional integrity of the corticospinal tract. TMS is a safe, non-invasive tool that can be used to stimulate primary motor cortex (M1). Stimulation of M1activates the corticospinal tract and induces responses in the contralateral muscles, visualised as a muscle twitch and recorded as motor evoked potentials (MEPs) using surface electromyography. TMS provides an objective and quantitative functional assessment of the motor cortex and its descending tracts after stroke. In the algorithm, if MEPs are present in the wrist extensor muscle, the patient has the potential to make a notable recovery of upper limb function within 12 weeks. While the recovery may not be complete, the patient will be able to use their affected upper limb in some activities of daily living by 12 weeks, even if they initially had a dense paresis and flaccid limb.
If MEPs cannot be elicited in the wrist extensors, diffusion-weighted MRI is used to assess the structural integrity of the posterior limbs of the internal capsules (PLICs). The PLICs contain the major white matter pathways between the motor and sensory cortices and the spinal cord. Damage to these pathways can be detected with diffusion-weighted MRI, which produces a measure of fractional anisotropy (FA). FA asymmetry between the PLICs of the lesioned and non-lesioned hemispheres can distinguish between patients with limited potential versus no potential for upper limb recovery .
1.3
The PREP algorithm
There is initial evidence in support of this sequential algorithmic approach. The predicting recovery potential (PREP) algorithm can accurately predict an individual’s potential for recovery of upper limb function . The PREP algorithm was tested with 40 sub-acute ischaemic stroke patients, to see whether it could predict individual patients’ subsequent upper limb function at 12 weeks after stroke. It had positive predictive power of 88%, negative predictive power of 83%, specificity of 88% and sensitivity of 73%. The PREP algorithm improves upon preceding algorithms proposed , by defining the FA asymmetry threshold between limited and no potential for recovery for patients at the sub-acute stage ( Fig. 1 ).
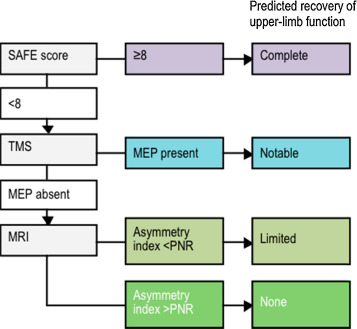
The PREP algorithm is designed for efficiency and economy, by starting with a simple bedside clinical assessment, and only using more advanced techniques if required to resolve uncertainty. The algorithm may be useful for setting realistic upper limb rehabilitation goals, and managing patient and therapist expectations. For example, rehabilitation goals for patients with notable recovery potential (a SAFE score below 8 and MEPs in the wrist extensors) could focus on strength, coordination and dexterity, while minimising compensation with the other hand. Rehabilitation for patients with no potential for upper limb recovery (a SAFE score below 8, no MEPs, and a high FA asymmetry) could focus on prevention of secondary complications such as shoulder subluxation and spasticity, and training compensation with the other hand for activities of daily living . The PREP algorithm could also be used to more accurately stratify patients in clinical trials. Further work is under way to test the PREP algorithm in patients with haemorraghic or previous stroke, and to explore the potential clinical and economic benefits of using the algorithm in clinical practice.
There have been further studies published since 2010 that explore the usefulness of clinical, neurophysiological and neuroimaging measures for predicting motor recovery after stroke. The following sections evaluate recent evidence for each of these types of measures.
1.4
Clinical assessment
The need for better predictive tools has been highlighted recently in a study by Nijland et al. . These authors reported the success rates of highly experienced therapists in predicting Action Research Arm Test (ARAT) score at six months based on assessment at 72 hours after stroke, and again at discharge from the acute stroke unit. Therapists were asked to predict future ARAT score within broad categories (< 10; 10–56; and 57). The predictions they made at 72 hours that 64 patients would have some upper limb function at six months (ARAT score 10–56) were no better than chance (53% incorrect). The authors proposed a computational prediction model based on clinical data, which improved prognosis statistically, but still led to an error rate of 42% for predicting outcomes within this range of function. This study demonstrates quite clearly that for many patients it is not yet possible to make accurate predictions based on clinical assessment alone. This study therefore supports the PREP algorithm approach, of adding neurophysiological and neuroimaging assessments if required to reduce uncertainty .
Clinical assessment may be more useful for predicting recovery of gait. Veerbeek et al. recently reported that sitting balance and lower limb strength 72 hours after stroke strongly predicted the recovery of independent gait at six months after stroke . These authors studied 154 patients and found that those who achieved both a full score on the Trunk Control Test and a Motricity Index score ≥ 25 within 72 hours, would recover independent gait by six months after stroke (Functional Ambulation Category ≥ 4). Conversely, patients who did not have sitting balance and some lower limb strength 72 hours after stroke had a 27% chance of achieving independent gait by six months after stroke. While these results are promising, the prediction model requires validation in a separate cohort of patients.
More generally, an absence of active movement in the upper or lower limb on initial assessment does not necessarily preclude motor recovery. Clinical assessment can be difficult in individuals with cognitive impairment, and the observed motor impairment on assessment may be due to lack of understanding rather than loss of movement of the limb. Neurophysiological measures provide an objective assessment of the functional integrity of descending motor pathways to the paretic limbs.
1.5
Neurophysiology
Transcranial magnetic stimulation can be used in diagnosis, prognosis, and treatment of motor deficits after stroke . The simple presence or absence of MEPs in the paretic limb can offer useful prognostic information. Motor evoked potential testing using TMS is more sensitive than conventional clinical assessment to detect residual corticospinal tract function. TMS can induce MEPs in around 30% of individuals with complete paralysis of the hand, and these patients’ potential for recovery would be otherwise be missed by conventional clinical assessment .
A more sophisticated way of using TMS to predict recovery after stroke has been reported by Di Lazzaro et al. . These authors used repetitive TMS to investigate motor cortex plasticity in 17 patients who had experienced stroke within the previous 10 days. The repetitive TMS protocol involved theta burst stimulation of the ipsilesional motor cortex, which is expected to facilitate its excitability. They found that the immediate effects of stimulation were correlated with modified Rankin Scale score at six months after stroke. Patients with the greatest neurophysiological response to the stimulation were more likely to have a better outcome. This study is important because it indicates that the plastic response to TMS may also be a useful predictor of recovery after stroke. However, the modified Rankin Scale is not particularly sensitive to recovery of motor function. The usefulness of this approach for predicting individual patients’ potential for motor recovery has yet to be tested.
1.6
Neuroimaging
Magnetic resonance imaging allows visualisation of the stroke lesion and its relationship to surrounding cortical and subcortical white and grey matter. Lesion volume information from MRI is not an independent predictor of motor recovery, and does not increase the accuracy of prediction models . More recent studies have confirmed that simple measures of lesion volume do not predict the extent of motor recovery in the upper limb and are not related to lower limb function at the chronic stage .
Diffusion tensor imaging allows the calculation of fractional anisotropy (FA) in each image voxel, which quantifies the directionality of water diffusion. The mean FA within specified areas of white matter can be used to quantify the structural damage caused by stroke to the white matter pathways. Diffusion tensor imaging can also be used to perform tractography, to reconstruct the corticospinal tract that descends from motor cortex, through the PLIC, to the spinal cord. The extent of overlap between the stroke lesion and the corticospinal tract , and the fractional anisotropy along the corticospinal tract , are both related to upper limb motor impairment and the response to therapy at the chronic stage of stroke.
A recent study by Puig et al. explored the usefulness of tractography for predicting motor recovery at the sub-acute stage . These authors rated the extent of corticospinal tract (CST) disruption in the motor cortex, premotor cortex, corona radiata, centrum semiovale and PLIC within 12 hours of symptom onset, and at three and 30 days after stroke, for 60 patients. They found that CST damage at the level of the PLIC, visualised within 12 hours of symptom onset, most strongly predicted upper and lower limb impairment 90 days after stroke. This highlights the value of assessing white matter integrity within the PLIC when predicting motor outcomes. However, it should be noted that motor impairment was evaluated with the NIH Stroke Scale, which is not particularly sensitive, and motor function was not assessed. In 2013, Puig et al. investigated the prediction of longer term motor outcomes . They used MRI to assess 70 patients within 12 hours of symptom onset, and at 3 and 30 days after stroke. They found that the ratio between the mean FA values in the ipsilesional and contralesional pons at 30 days after stroke was the only independent predictor of motor outcome at two years after stroke, measured with the Motricity Index. This study again confirms the importance of the CST for recovery of motor function, however the predictive power of FA asymmetry within the PLIC wasn’t evaluated.
The usefulness of FA measures for predicting motor outcomes after subcortical haemorrhagic stroke have recently been evaluated by Koyama et al. . These authors obtained MRI scans from 32 patients within three weeks of stroke. The ratio of FA values between hemispheres in the cerebral peduncles, and in the corona radiata/PLIC, were related to upper and lower limb strength at one month measured with the MRC scale. The ratio of FA values in the cerebral peduncles was more strongly related to motor strength than the ratio obtained from the corona radiata/PLIC. These results indicate that motor outcomes after subcortical stroke may be more accurately predicted by FA measures made distal to the stroke lesion. However, the MRC scale is not particularly sensitive, and does not reflect recovery of function in the upper or lower limb. A recent study of patients at the chronic stage found that FA asymmetry in the PLIC was strongly related to walking ability , adding further support to the use of this neuroimaging measure for predicting subsequent recovery of function in both the lower and upper limbs.
Functional neuroimaging tools can also be used to examine cortical activation after stroke. The basis of functional MRI (fMRI) is that the blood-oxygenation level dependent (BOLD) signal detects changes in haemoglobin levels as a proxy for neuronal activity. The degree of cortical activation, and the regions activated, is highly variable post-stroke. Very few studies have examined the relationship between fMRI measures and subsequent recovery of motor function after stroke. Marshall et al. obtained fMRI scans from 23 patients within days of stroke . They related the average pattern of cortical activity during repetitive closure of the paretic hand to the change in upper limb motor impairment over the following three months, measured with the Fugl-Meyer scale. They found that there was a pattern of cortical activity that was positively related to improve upper limb movement for this group of patients. This is a useful first step towards using fMRI measures to predict motor recovery. It remains to be seen whether fMRI measures can be used to make accurate predictions for individual patients, and whether fMRI measures are superior to structural measures of white matter tract integrity. A more recent study of patients between three and nine months after stroke found that the integrity of the descending white matter tracts in the PLIC was more strongly correlated with upper limb function than fMRI measures .
1.7
Beyond the corticospinal tract
The degree of damage to the corticospinal tract is linked closely with motor function recovery, but other white matter pathways may also have a role. The integrity of alternate motor fibres and transcallosal fibers may also be important for recovery. It is hypothesised, based primarily on non-human imaging data, that these alternate motor fibres consist of the corticorubrospinal pathway and the corticoreticulospinal pathway ( Fig. 2 ). A recent study of 18 patients at the chronic stage of stroke reported that the structural integrity of both the ipsilesional corticospinal tract and the ipsilesional alternate motor fibres is strongly related to motor function of the upper limb, measured with the Wolf Motor Function Test (WMFT) . A similar study of 15 patients at the chronic stage reported that FA measured in the corpus callosum, connecting the primary motor cortices of each hemisphere, was also related to motor function of the upper limb measured with the WMFT, and the response to therapy .
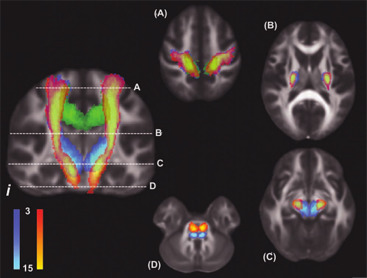
Contralesional descending motor tracts may also have a role to play in the control of the proximal muscles of the paretic limb. The potential contribution of contralesional motor pathways to walking has recently been investigated. Jang et al. examined the corticospinal and corticoreticular tracts in both hemispheres of 54 chronic stroke patients . They found that the number of fibres in the contralesional corticoreticular tract was related to walking ability, assessed with the Functional Ambulation Category. Other alternate motor pathways such as the contralesional corticoreticulospinal pathway may contribute to proximal upper limb recovery, particularly when the ipsilesional corticospinal tract has sustained significant damage .
Together, these recent studies indicate that the prediction of motor recovery after stroke could usefully consider the integrity of motor pathways in both hemispheres, including corticorubrospinal and corticoreticulospinal tracts. However, studies to date have focused on patients at the chronic stage of stroke. The usefulness of examining other motor pathways at the sub-acute stage remains to be explored.
1.8
Combined measures
The PREP algorithm is currently the only approach that combines clinical, neurophysiological and neuroimaging measures in a sequential way. More recent studies have compared the prognostic accuracy of TMS and diffusion-weighted MRI measures. Jang et al. used TMS and tractography to study 54 patients with intracerebral haemorrhage and severe motor weakness. The TMS and MRI measures were made within four weeks of stroke, and were related to the upper limb Motricity Index at six months after stroke . As expected, patients in whom MEPs could be elicited in the paretic upper limb, and with an intact corticospinal tract visualised with tractography, had better motor outcomes. These authors also report that TMS had a higher positive predictive power, while tractography had a higher negative predictive power, and this finding was replicated by these authors in a subsequent study . These studies confirm that neuroimaging is particularly useful for identifying which patients with no MEPs also have no potential for motor recovery, in line with the PREP algorithm.
1.9
Conclusions and future directions
Combining neurophysiological and neuroimaging measures can improve predictive power . However obtaining these measures for all patients may not be practical or economical. The PREP algorithm recommends sequentially combining measures, beginning with a simple bedside clinical assessment. TMS and MRI measures are only made as required to resolve uncertainty. Research in this area since the algorithm was first proposed generally supports this approach. The PREP algorithm is an example of how accurate predictions can be made for individual patients, and rehabilitation plans can then be tailored appropriately. Algorithms such as PREP can also strengthen the design of randomised control trials of new therapies that aim to improve motor outcomes after stroke. For example, determining an individual patient’s corticospinal tract integrity predicts response to non-invasive brain stimulation of M1 at the chronic stage . This type of information could be deemed essential for stratification of patients, especially in studies with small numbers of patients, as is common with rehabilitation trials. This stratification procedure has also been recognised to be critically important in trials of new experimental therapies . Currently there is no predictive algorithm for recovery of lower limb function, although recent evidence indicates that MEP and FA measures could be of some use. Further research is needed to determine if such techniques can be used and combined early after stroke to improve prognosis of gait recovery.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
2
Version française
2.1
Introduction
L’accident vasculaire cérébral (AVC) représente la troisième cause de handicap acquis de l’adulte dans les pays industrialisés . Aux États-Unis, on compte environ 800 000 AVC annuellement, et un tiers des survivants auront des séquelles responsables de handicap . La capacité de la personne à assumer les activités de la vie quotidienne après AVC dépend de la récupération de la fonction motrice, tout spécialement pour le membre supérieur .
La récupération de la fonction motrice est caractérisée par la capacité de l’individu à accomplir de manière similaire les mouvements faisant appel aux mêmes effecteurs et schémas d’activation musculaire qu’avant l’AVC . La récupération est très variable dans les premiers jours après l’AVC quand la rééducation fonctionnelle commence, rendant difficile une estimation précise de l’étendue de la récupération motrice qui ne sera effective que plusieurs mois après l’AVC en fin de rééducation. La capacité à prédire le potentiel de récupération motrice d’un individu pourrait se révéler utile car cela permettrait de mettre en place une rééducation sur mesure, de prendre en charge les attentes des patients et des thérapeutes, et pourrait résulter en une utilisation plus efficace des ressources de santé publique.
Cette revue détaille les développements ayant eu lieu depuis 2010, lorsque nous avions proposé un algorithme prédictif du potentiel de récupération de la fonction motrice du membre supérieur pour chaque patient . Dans ce travail, nous identifions de nouvelles études sur les facteurs clés prédictifs de la récupération motrice post-AVC, qui soutienne l’algorithme proposé et qui pourraient être extrapolées au membre inférieur.
2.2
Une approche multimodale
Actuellement, trois méthodes principales ont été identifiées pour évaluer la capacité de récupération motrice dans les jours qui suivent l’AVC : les échelles d’évaluation clinique, les évaluations neurophysiologiques et les techniques de neuroimagerie. En 2010, nous proposions un algorithme séquentiel pour combiner ces trois méthodes afin d’établir un pronostique fiable de la récupération motrice du membre supérieur du patient au début de sa rééducation fonctionnelle, durant la phase subaiguë de l’AVC. L’algorithme commence par une évaluation clinique complète dans les 72 heures après l’apparition des symptômes de l’AVC . L’abduction de l’épaule et la force d’extension des doigts sont notées sur 5 sur l’échelle MRC (Medical Research Council) et ces notes sont ensuite additionnées pour produire un score shoulder abduction, finger extension (SAFE), dont le score maximal est 10. Les patients ayant un score SAFE de 8 ou plus ont le potentielpour une récupération motrice complète, ou presque complète dans les 12 semaines qui suivent leur AVC.
Les patients avec un score SAFE en dessous de 8 passent une évaluation neurophysiologique dans la semaine suivant l’apparition des symptômes. La stimulation magnétique transcranienne ( transcranial magnetic stimulation [TMS]) est utilisée pour évaluer l’intégrité fonctionnelle du faisceau corticospinal. La TMS est une méthode sans danger et non invasive utilisée pour stimuler le cortex moteur primaire (M1). La stimulation du M1 active le faisceau corticospinal qui induit une réponse des muscles controlatéraux, cette réponse est visualisée comme un tressautement musculaire, elle est enregistrée en tant que potentiels moteurs évoqués (PME) à l’aide de l’électromyographie de surface. La TMS fournit une évaluation fonctionnelle quantitative et objective après l’AVC du cortex moteur et de ses faisceaux descendants. Dans l’algorithme, si des PME sont présents dans les extenseurs du poignet, le patient montre un potentiel pour une récupération motrice notable du membre supérieur dans les 12 semaines. Même si la récupération n’est pas complète, le patient sera capable d’utiliser son membre supérieur lésé dans certaines activités de la vie quotidienne au bout de 12 semaines après l’AVC, même si initialement il existait une parésie sévère et une flaccidité du membre.
Si les PEM ne sont pas retrouvés dans les extenseurs du poignet, on a recours à l’IRM de diffusion pour évaluer l’intégrité structurelle des bras postérieurs des capsules internes (BPCI). Les BPCI contiennent les principaux faisceaux de la substance blanche reliant les cortex moteurs et sensitifs et la moelle épinière. Une lésion de ces faisceaux peut être détectée à l’aide de l’IRM de diffusion qui permet de mesurer la fraction d’anisotropie (FA). L’asymétrie FA entre les BPCI de l’hémisphère sain et l’hémisphère lésé peut différentier les patients ayant un potentiel limité de récupération fonctionnelle du membre supérieur de ceux n’ayant aucun potentiel de récupération .
2.3
L’algorithme PREP
Des preuves initiales soutiennent cette approche algorithmique séquentielle. L’algorithme predicting recovery potential (PREP) peut prédire de manière fiable le potentiel individuel de récupération motrice du membre supérieur des patients . L’algorithme PREP a été testé chez 40 patients en phase subaiguë d’AVC ischémique, afin d’évaluer sa capacité à prédire la motricité fonctionnelle du membre supérieur des patients 12 semaines après l’AVC. Les résultats montrent un pouvoir prédictif positif de 88 %, un pouvoir prédictif négatif de 83 %, une spécificité de 88 % et une sensibilité de 73 %. L’algorithme PREP est une amélioration par rapport aux autres algorithmes proposés , il permet de définir le seuil d’asymétrie FA entre un potentiel limité et aucun potentiel de récupération chez le patient en phase subaiguë d’AVC ( Fig. 1 ).