Abstract
Osteoarthritis (OA) is the most common form of joint disease. This review aimed to consolidate the current evidence that implicates the inflammatory process in the attenuation of synovial lubrication and joint tissue homeostasis in OA. Moreover, with these findings, we propose some evidence for novel therapeutic strategies for preventing and/or treating this complex disorder. The studies reviewed support that inflammatory mediators participate in the onset and progression of OA after joint injury. The flow of pro-inflammatory cytokines following an acute injury seems to be directly associated with altered lubricating ability in the joint tissue. The latter is associated with reduced level of lubricin, one of the major joint lubricants. Future research should focus on the development of new therapies that attenuate the inflammatory process and restore lubricin synthesis and function. This approach could support joint tribology and synovial lubrication leading to improved joint function and pain relief.
1
Introduction
Osteoarthritis (OA) has long been considered a “wear and tear” disease. Traditionally, the etiology of OA has been linked to increased mechanical overload on weight bearing joints, anatomical joint incongruence, and fragility of articular cartilage . However, this concept is gradually being challenged as evidence accumulates to support an “inflammatory” basis of OA.
The capacity for joint repair gradually diminishes with aging. The articular cartilage component of the joint is often damaged in focal or more extensive areas after joint injury. Cartilage is a connective tissue that is neither vascularized nor innervated and therefore cannot respond to acute injuries with the usual cycle of reparative responses . Chondrocytes, the unique cells that are present in cartilage, are sparsely distributed within the tissue and have a low reparative capacity because they have very low metabolic activity.
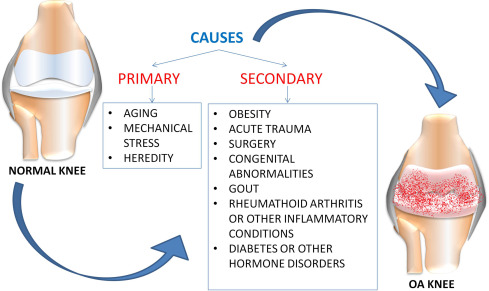
OA is a complex disease with a multifactorial etiology that includes aging, synovitis, “low-grade” systemic inflammation, obesity, prior joint injuries, gender, genetic factors and metabolic syndrome among the most prominent risk factors for development and progression ( Fig. 1 ). Another fundamental aspect of the OA pathophysiological process is the reduced boundary-lubricating ability of synovial fluid. This aspect is associated with reduced level of lubricin, one of the major joint lubricants .
In this review, we discuss the most important findings regarding the inflammatory process and the altered lubricating ability in the joint tissue following acute injury, to highlight a possible cross-link between these 2 pathological aspects of OA, a complex disease. The key observations in this review should provide further motivation for studying the link between these 2 important features of OA, laying the foundation for novel therapeutic approaches and innovative treatments.
2
Inflammatory theory of OA
The “inflammatory theory” of OA onset is certainly not a recent concept. Indeed, in a paper published in 1975, George Ehrlich described a cohort of predominantly menopausal females presenting a deforming and inflammatory OA, some of whom showed changes characteristic of rheumatoid arthritis (RA) . This was probably the first paper that emphasized inflammation as a key component of OA. Although the original observations were published more than 40 years ago, the importance of Ehrlich’s findings has not been fully appreciated until recently. The major players in OA research have realized the importance of this aspect and proposed a connection between inflammation, synovitis and structural changes in OA. The advent of molecular biology and its introduction to bone and joint research dates back to the 1990s. Numerous soluble mediators of inflammation, such as cytokines and prostaglandins, were discovered and found to be associated with increased production of matrix metalloproteinases (MMPs), primary enzymes responsible for cartilage degradation . More recent data indicate subchondral bone, cartilage and synovium as a source of inflammatory mediators in OA progression and cartilage degeneration . These data emphasize the complexity of the disease, implicating the entire joint as an organ and not just cartilage as a joint tissue. Such findings are supported by a recent discovery of the effect of pro-inflammatory cytokines on the reduced production of lubricin and the consequent decreased boundary-lubricating ability of synovial fluid in OA joints, which suggests the important role of lubricin in the development of this complex disease .
2.1
Inflammatory cytokines
A primarily destructive impact on cartilage is the effect of inflammatory cytokines associated with biomechanical factors. The latter have a multilevel impact on joint tissues, involving premature aging, chondrocyte apoptosis and decreased synthesis of key components of extracellular matrix. Inflammatory cytokines also contribute to increased synthesis of many proteolytic enzymes, responsible for cartilage degradation and determine the reduced lubricating ability of synovial fluid. Among the inflammatory cytokines determining the loss of metabolic homeostasis of joint tissues by promoting catabolic and destructive processes, those with the greatest effect are interleukin 1β (IL-1β), tumor necrosis factor α (TNF-α), IL-6, IL-15, IL-17, and IL-18 ( Fig. 2 and Table 1 ) .
2.2
Synovitis
Synovitis is a critical feature of OA and many studies have focused on this condition as a key driver of the disease process. Synovitis is defined as the local inflammation of synovial membrane, usually painful, characterized by joint swelling due to synovial thickening or effusion. Synovial inflammation occurs frequently after traumatic joint injury and is associated with increased pain and dysfunction . It probably occurs as degraded cartilage fragments and extracellular matrix macromolecules are released into the joint and contact the synovium. Synovial cells react to the release of these fragments/molecules and become activated, thereby producing inflammatory mediators . The latter stimulate chondrocytes in the superficial layer of cartilage and the synovium itself to synthesize MMPs and other matrix-degrading enzymes that increase cartilage degradation. These mediators are also responsible for synovial angiogenesis and increased synthesis of inflammatory cytokines and MMPs by synovial cells themselves, for a vicious cycle . Clearly, the described events affect the lubricating ability of the joint as confirmed by Jay et al. in a study of the lubricating ability of aspirated synovial fluid from patients with knee joint synovitis. The study demonstrated the non-lubricating bearing and increased catabolism of collagen II in synovial fluid aspirates of these patients, so collagen II may play a fundamental role in acute cartilage destruction ultimately resulting in post-traumatic OA .
2.3
Inflammaging
Age is the most important risk factor for OA onset. The aging process does not necessarily relate to the passage of time but depends also on our lifestyle. Indeed, 2 different types of cellular senescence are replicative and stress-induced. The former is associated with an arrest in cell-cycle progression, resulting from a natural telomere shortening process and found in cells in older adults. The latter is independent of telomere length and is associated with several kinds of stresses, especially oxidative stress and inflammatory processes established during OA onset . “Inflammaging” refers to low-grade inflammation that occurs during physiological aging. According to this concept, the inflammatory process, as well as all events closely linked to it, contributes to chondrocyte senescence, which results in the age-related degradation of cartilage, subchondral bone, and synovium, thereby determining the early development of OA . Interestingly, the link between inflammation and aging appears to be interdependent because it determines the early senescence of chondrocytes and also appears to be its direct consequence. Indeed, activity is impaired in senescent chondrocytes as compared with normal chondrocytes, with evidence of the “senescent secretory phenotype”, characterized by increased expression of genes encoding for inflammatory cytokines such as IL-6, IL-1β and several members of the MMP family . The increased expression of both advanced glycation end-products (AGEs) and AGE receptors (RAGEs) in OA chondrocytes has been associated with dysregulated signalling pathways, altered synthetic activity and enhanced sensitivity to cytokines and chemokines, which in turn trigger the expression of MMPs and other inflammatory mediators . Interestingly, lubricin production in aged rats appeared to be decreased, so chondrocyte senescence may have an important role in the lubricating properties of cartilage tissue . This observation is probably a direct consequence of the above suggested strict link between aging and inflammation.
2.4
Chitinases
Lately, much interest has been given to some members of the family of chitinases such as chitotriosidase (CHIT1) and chitinase 3-like-1 (CHI3L1) involved in OA pathophysiology. Elevated levels of these proteins have been reported in several chronic inflammatory and degenerative disorders . In our recent study, we observed increased expression of these proteins in a rat model of OA. Their production has been closely related to inflammatory processes and pro-inflammatory cytokines, and their overexpression was suggested to be involved in cartilage remodelling and degradation processes in OA joints . Moreover, in another recent study, we demonstrate an inverse proportional relation of the expression of CHI3L1 and lubricin in normal and osteoarthritic rat articular cartilage. Levels of lubricin increased in normal cartilage and decreased in OA cartilage and levels of CHI3L1 increased in OA cartilage and decreased in normal cartilage. These 2 glycoproteins may be functionally associated with the development of OA, which again underlines the important link between the inflammation and lubricating properties of articular cartilage tissue in OA onset .
2
Inflammatory theory of OA
The “inflammatory theory” of OA onset is certainly not a recent concept. Indeed, in a paper published in 1975, George Ehrlich described a cohort of predominantly menopausal females presenting a deforming and inflammatory OA, some of whom showed changes characteristic of rheumatoid arthritis (RA) . This was probably the first paper that emphasized inflammation as a key component of OA. Although the original observations were published more than 40 years ago, the importance of Ehrlich’s findings has not been fully appreciated until recently. The major players in OA research have realized the importance of this aspect and proposed a connection between inflammation, synovitis and structural changes in OA. The advent of molecular biology and its introduction to bone and joint research dates back to the 1990s. Numerous soluble mediators of inflammation, such as cytokines and prostaglandins, were discovered and found to be associated with increased production of matrix metalloproteinases (MMPs), primary enzymes responsible for cartilage degradation . More recent data indicate subchondral bone, cartilage and synovium as a source of inflammatory mediators in OA progression and cartilage degeneration . These data emphasize the complexity of the disease, implicating the entire joint as an organ and not just cartilage as a joint tissue. Such findings are supported by a recent discovery of the effect of pro-inflammatory cytokines on the reduced production of lubricin and the consequent decreased boundary-lubricating ability of synovial fluid in OA joints, which suggests the important role of lubricin in the development of this complex disease .
2.1
Inflammatory cytokines
A primarily destructive impact on cartilage is the effect of inflammatory cytokines associated with biomechanical factors. The latter have a multilevel impact on joint tissues, involving premature aging, chondrocyte apoptosis and decreased synthesis of key components of extracellular matrix. Inflammatory cytokines also contribute to increased synthesis of many proteolytic enzymes, responsible for cartilage degradation and determine the reduced lubricating ability of synovial fluid. Among the inflammatory cytokines determining the loss of metabolic homeostasis of joint tissues by promoting catabolic and destructive processes, those with the greatest effect are interleukin 1β (IL-1β), tumor necrosis factor α (TNF-α), IL-6, IL-15, IL-17, and IL-18 ( Fig. 2 and Table 1 ) .
2.2
Synovitis
Synovitis is a critical feature of OA and many studies have focused on this condition as a key driver of the disease process. Synovitis is defined as the local inflammation of synovial membrane, usually painful, characterized by joint swelling due to synovial thickening or effusion. Synovial inflammation occurs frequently after traumatic joint injury and is associated with increased pain and dysfunction . It probably occurs as degraded cartilage fragments and extracellular matrix macromolecules are released into the joint and contact the synovium. Synovial cells react to the release of these fragments/molecules and become activated, thereby producing inflammatory mediators . The latter stimulate chondrocytes in the superficial layer of cartilage and the synovium itself to synthesize MMPs and other matrix-degrading enzymes that increase cartilage degradation. These mediators are also responsible for synovial angiogenesis and increased synthesis of inflammatory cytokines and MMPs by synovial cells themselves, for a vicious cycle . Clearly, the described events affect the lubricating ability of the joint as confirmed by Jay et al. in a study of the lubricating ability of aspirated synovial fluid from patients with knee joint synovitis. The study demonstrated the non-lubricating bearing and increased catabolism of collagen II in synovial fluid aspirates of these patients, so collagen II may play a fundamental role in acute cartilage destruction ultimately resulting in post-traumatic OA .
2.3
Inflammaging
Age is the most important risk factor for OA onset. The aging process does not necessarily relate to the passage of time but depends also on our lifestyle. Indeed, 2 different types of cellular senescence are replicative and stress-induced. The former is associated with an arrest in cell-cycle progression, resulting from a natural telomere shortening process and found in cells in older adults. The latter is independent of telomere length and is associated with several kinds of stresses, especially oxidative stress and inflammatory processes established during OA onset . “Inflammaging” refers to low-grade inflammation that occurs during physiological aging. According to this concept, the inflammatory process, as well as all events closely linked to it, contributes to chondrocyte senescence, which results in the age-related degradation of cartilage, subchondral bone, and synovium, thereby determining the early development of OA . Interestingly, the link between inflammation and aging appears to be interdependent because it determines the early senescence of chondrocytes and also appears to be its direct consequence. Indeed, activity is impaired in senescent chondrocytes as compared with normal chondrocytes, with evidence of the “senescent secretory phenotype”, characterized by increased expression of genes encoding for inflammatory cytokines such as IL-6, IL-1β and several members of the MMP family . The increased expression of both advanced glycation end-products (AGEs) and AGE receptors (RAGEs) in OA chondrocytes has been associated with dysregulated signalling pathways, altered synthetic activity and enhanced sensitivity to cytokines and chemokines, which in turn trigger the expression of MMPs and other inflammatory mediators . Interestingly, lubricin production in aged rats appeared to be decreased, so chondrocyte senescence may have an important role in the lubricating properties of cartilage tissue . This observation is probably a direct consequence of the above suggested strict link between aging and inflammation.
2.4
Chitinases
Lately, much interest has been given to some members of the family of chitinases such as chitotriosidase (CHIT1) and chitinase 3-like-1 (CHI3L1) involved in OA pathophysiology. Elevated levels of these proteins have been reported in several chronic inflammatory and degenerative disorders . In our recent study, we observed increased expression of these proteins in a rat model of OA. Their production has been closely related to inflammatory processes and pro-inflammatory cytokines, and their overexpression was suggested to be involved in cartilage remodelling and degradation processes in OA joints . Moreover, in another recent study, we demonstrate an inverse proportional relation of the expression of CHI3L1 and lubricin in normal and osteoarthritic rat articular cartilage. Levels of lubricin increased in normal cartilage and decreased in OA cartilage and levels of CHI3L1 increased in OA cartilage and decreased in normal cartilage. These 2 glycoproteins may be functionally associated with the development of OA, which again underlines the important link between the inflammation and lubricating properties of articular cartilage tissue in OA onset .
3
Inflammation and lubricin synthesis
3.1
Lubricin
The biology of OA has been attributed to changes in lubrication at the surface of articular cartilage. This aspect of the pathophysiological process of OA has not been entirely understood. Lubricin is a surface-active mucin-like glycoprotein, encoded by the proteoglycan 4 ( PRG4 ) gene, specifically synthesized by chondrocytes located at the surface of articular cartilage . As a lubricating glycoprotein, lubricin is produced in synovial fluid , menisci , the superficial layer of articular cartilage , tendons , the temporomandibular joint disc and the periodontal ligament . Also called superficial zone protein (SZP), lubricin has been reported to be a proteoglycan, specifically PRG4. Lord et al. demonstrated that lubricin in human synovial fluid is a heterogeneous population with both glycoprotein and proteoglycan forms . PRG4 has been identified as megakaryocyte stimulating factor (MSF); the expression of human and mouse PRG4 genes was found to be similar and was found in cartilage and also liver, heart, lung, and bone . Ludwig et al. demonstrated lower levels of PRG4 and reduced boundary lubrication properties in the synovial fluid of human patients with symptomatic OA. In contrast, and perhaps unexpectedly, Neu et al. found elevated levels of SZP in patients with advanced OA, so SZP may be ineffective in reducing joint friction in the boundary lubrication mode in advanced OA, where other mechanisms may dominate the observed tribological behaviour. Lubricin contains multiple protein domains. The largest central mucin-like domain (high content of proline, serine and threonine) consists of imperfectly repeated sequences of EPATTPK, which provide the scaffold for O-glycosylation . This domain contains the C-terminal haemopexin domain and 2 somatomedin domains at the N-terminus. The boundary-lubricating ability of lubricin has been attributed to its O-glycans, which affect physical properties such as high viscosity and low friction . Glycomic studies have shown that lubricin presents abundant sialylated and unsialylated core 1 oligosaccharides and several sialylated, fucosylated and sulfated core 2 oligosaccharides .
3.2
Lubricin and OA
Lubricin is critical to normal joint function, providing boundary lubrication of congruent articular surfaces under high contact pressure and near-zero sliding speed. Furthermore, it has an important role in preventing chondrocyte apoptosis and in synovial cell adhesion and proliferation . Lubricin-knockout mice show clinical and radiologic signs of joint disease and histologic abnormalities in their articulating joints with increasing age. The most important features are synovial hyperplasia and subintimal fibrosis, proteinaceous deposits on the cartilage surface, irregular cartilage surface and endochondral growth plates, and abnormal calcification in tendon sheaths and osteophytes . Furthermore, decreased synthesis of lubricin has been observed in several studies of both OA joints and post-traumatic OA . In one study , joint friction and cellular apoptosis was greater in lubricin-knockout than wild-type mice. The addition of lubricin in the in vitro bovine explant cartilage-on-cartilage bearing system significantly lowered the coefficient of friction and chondrocyte apoptosis in superficial layers of cartilage, thereby confirming its crucial role in preventing cartilage degeneration . Supplementing lubricin by intra-articular injection improved weight bearing in studies measuring hind limb force. Lubricin reduced the severity of post-traumatic OA and level of urinary C-terminal cross-linked telopeptide type II collagen (CTX-II), without affecting Osteoarthritis Research Society International (OARSI) score . Overproduction of lubricin in transgenic mice reduced the severity of both age-related and post-traumatic OA. This reduction was due to lubricin inhibiting the expression of genes involved in cartilage catabolism and chondrocyte hypertrophy by upregulating hypoxia-inducible factor 3α (HIF-3α), a negative regulator of HIF-1α and HIF-2α, responsible for catabolic and anabolic activity that promotes OA . The early stages after anterior cruciate ligament injury are characterized by changes in levels of sulfated glycosaminoglycans (sGAGs). The significantly increased sGAG and aquaporin levels in synovial fluid after joint injury may indicate articular cartilage damage . Proteoglycan turnover may be increased with low lubricin values because decreased levels have been consistently associated with high sGAG concentrations . Lubricin levels then appear to recover within 1 year after injury ; even if once cartilage tissue damage is initiated, it appears to persist over time. These findings support that the initial reduction in lubricin synthesis may initiate a cascade of events leading, over time, to the onset of OA. However, this observation needs further studies to support this hypothesis.
3.3
OA-related lubricin reduction mechanisms
The principal mechanism proposed for the reduction of lubricin synthesis is the degradation activity of neutrophil-derived enzymes and inflammatory mediators present in post-traumatic SF . Some cytokines (IL-1β, TNF-α and IL-6) are associated with the upregulation of proteolytic enzymes such as procathepsin-B, neutrophil elastase and MMPs, which degrade lubricin and lead to loss of synovial fluid chondroprotection, especially in the early stages after injury ( Fig. 3 ). The proteolytic activity seems to be an important link between the inflammatory process and the decreased synovial fluid lubricating ability, which suggests an intimate correlation between these two pathological aspects of OA. In the later stages of injury, other factors such as joint utilization and loss of intra-articular surface congruence may contribute to potentiating this damage. This notion may complicate an understanding of the real sequence of catabolic events that occur during OA onset .







