Biology
- ▪
Motion aids healing and reduces adhesions.
- ▪
There is no evidence that loading, in the absence of motion, is helpful, or that, once the tendon is moving, more loading helps healing.
- ▪
We do know that loading may lead to failure of the repair.
Safe Zone
- ▪
Enough loading to initiate motion
- ▪
Not enough to risk the repair
Future Surgical Methods
- ▪
Gaps: Lubricated graft
- ▪
Slow healing: Cell-rich patch between tendon ends, with or without cytokines
- ▪
Stronger repairs: Newer sutures
Significance of Tendon Injuries
Upper extremity injuries are common, representing approximately one third of all traumatic injuries. Tendon injuries are among the most severe upper extremity injuries. The number of tendon injuries is difficult to quantify because epidemiologic studies have not been done, but estimates suggest that roughly 40,000 inpatient tendon repairs are done each year in the United States. A much larger number of tendon surgeries are done on an outpatient basis. More importantly, these injuries occur almost exclusively in a young, working-age population and result in considerable disability. The typical tendon injury requires 3 to 4 months of rehabilitation, during which time the affected hand is unavailable for work use. Failure rates or residual impairment remain disturbingly high, in the 20% to 30% range in most series, despite ongoing attention to the problem. From 1976 to 1999, consistently between 7% and 8% of the articles in the Journal of Hand Surgery focused on tendon injuries. Despite this evident importance and ongoing interest, translation of research results into meaningful clinical improvements have been limited. By most accounts, the most significant improvement in tendon rehabilitation remains the institution of early passive motion therapy by Kleinert in the early 1970s. Since then, quality improvements have been incremental. Tendon rupture rates continue to be cited at an incidence of 5% to 10%. These failures require complex secondary tendon reconstruction surgeries. Better methods for improving intrinsic tendon healing and minimizing tendon adhesions are still needed so we can improve upon clinical outcomes, with the ultimate goal being the production of an adhesion-free tendon repair.
Tendon Healing
The extracellular matrix (ECM) is the principal component of tendon tissue and is responsible for its material properties. The major constituents of the ECM are type I collagen; proteoglycans, principally decorin, but also aggrecan in the gliding regions; fibronectin; and elastin. This matrix is synthesized by tendon cells, or tenocytes ( Fig. 34-1 ). These cells are surrounded by the dense matrix; thus, although they are metabolically active, they do not participate much in the tendon-healing process. Instead, undifferentiated cells in the epitenon do the heavy lifting for tendon healing, and proliferating, migrating into the gap between the tendon ends, and finally uniting the cut tendon ends , ( Fig. 34-2 ). Unfortunately, this process presents a bit of a dilemma; if these same cells migrate away from the tendon, toward the tendon sheath, they form adhesions that restrict tendon motion. Often this is indeed the case, as the relatively ischemic tendon is surrounded by better vascularized tissue, which sends out vascular buds under the stimulation of vascular endothelial growth factor (VEGF).


After tendon injury, the ECM undergoes significant changes due to synthesis of new elements, such as type III collagen, by the tenocytes, degradation of existing elements by various matrix metalloproteinases (MMP), and remodeling of the resulting combination, under the influence of cytokines such as transforming growth factor beta (TGF-β) as well as mechanical forces. Manipulation of these processes, to augment their action between the tendon ends while reducing them at the tendon’s gliding surface, is the goal of much research, as described later.
Pharmacologic Manipulation of Tendon Healing
Various pharmacologic agents have been used in the past in an attempt to modify adhesion formation. Steroids, antihistamines, and β-aminoproprionitrile have not been shown to decrease scar formation clinically. , Ibuprofen and indomethacin, however, have been found to have a small beneficial effect.
The ideal pharmacologic agent should have no systemic side effects, should be limited to a single application, and should be directed at growth factor expression and ECM production. Such a drug may be 5-fluorouracil (5-FU), an antimetabolite used not only as a cancer chemotherapeutic agent but also to prevent adhesions in glaucoma filtration surgery. The exposure of a surgical field to 5-FU produces a focal inhibition of scarring. Blumenkranz and colleagues have found that 5-FU inhibits the proliferation of fibroblasts in cell cultures and reduces retinal scarring. , Single exposures to 5-FU, for as short duration as 5 minutes, can have antiproliferative effects on fibroblasts for several days. The suppression of fibroblast proliferation has been observed for up to 36 hours without signs of cell death. , This time frame may be adequate to inhibit tendon adhesions prior to beginning postoperative motion protocols. Reversible prolonged inhibition of fibroblast function is attributed to the drug’s inhibition of DNA and messenger RNA (mRNA) synthesis through thymidylate syntheses. More importantly, these effects appear to be focal to the site of application and titratable in terms of length of action. A 5-minute exposure to 5-FU has been shown to significantly decrease postoperative flexor tendon adhesions in chicken and rabbit models. , This beneficial effect is felt to be due to the down-regulation of TGF-β and modulation of MMP-2 and MMP-9 production. , The effect on surface lubrication is unknown. No adverse effect was noted on tendon healing in these studies. It is presumed therefore that the topical 5-FU does not penetrate to affect the cells below the tendon surface. Topical 5-FU may well have a role in improving the outcomes in selected cases of tenolysis.
Growth Factors
Growth factors are the chemical signals that direct the migration and proliferation of the tendon fibroblast during the healing process. The role of growth factors has been examined extensively in cutaneous wounds and other soft tissue processes, yet we are only beginning to know the specifics involved in flexor tendon healing. , The factors that appear to be involved include TGF-β, platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), insulin-like growth factor (IGF), epidermal growth factor (EGF), and VEGF. These same growth factors have also been shown to optimize tissue-engineered constructs used for tendon repair. Growth differentiation factor-5 (GDF-5), a member of the TGF-β superfamily, has also been shown to accelerate tendon healing in multiple animal models.
TGF-β stimulates the formation of the ECM. It signals fibroblasts to produce collagen and fibronectin, decreases protease production, and increases the formation of integrins, which promote cellular adhesions and matrix assembly. In normal tissue, TGF-β becomes inactivated once wound healing is complete; however, it may remain active in tendon adhesion formation, continuing the cycle of matrix accumulation. , Excessive expression of TGF-β is detrimental to many tissues, resulting in tissue fibrosis in the heart, kidney, and liver. Modulation of TGF-β has been reported to reduce the fibrotic process in glomerulonephritis, dermal wounds, and arthritis as well as decreasing peritendinous adhesions in a rabbit tendon model. TGF-β levels can remain elevated for up to 8 weeks after tendon injury. ,
Neuropeptides may also play a role in tendon healing. During the early phases of healing, tendons exhibit nerve fiber ingrowth. This nerve ingrowth is associated with the temporal release of substance P (SP). SP promotes tendon regeneration through the stimulation and proliferation of fibroblasts. Further studies have found that tendon motion helps to modulate the release of SP. The injection of SP into the peritendinous region of ruptured rat tendons improves healing and increases tendon strength. Similarly, GDF-5 has a potential to stimulate bone marrow-derived stem cell (BMSC) proliferation and regulate BMSC differentiation to tenocytes. Recent experiments have shown a beneficial effect of GDF-5 on tendon healing as well.
Tendon Grafting
At one time, most flexor tendon injuries were treated with tendon grafts, , but today primary repair is used almost exclusively, with grafts being used primarily to reconstruct otherwise unbridgeable tendon gaps. This is a good thing, since tendons in the hand are intrasynovial and have a specialized gliding surface, whereas most tendon grafts, such as the palmaris or plantaris, are extrasynovial and have no such specialized surface. , The result is much more adhesion formation than would be the case if intrasynovial grafts were available. As noted later, in the future it may be possible to engineer such grafts to reduce friction and improve healing.
Augmentation of Intrinsic Tendon Healing with Stem Cells
Healing of flexor tendons in zone 2 depends on the ability of the injured tendon to recruit fibroblasts and other cellular components to the site of injury. Normally these are circulating or locally derived undifferentiated (i.e., stem) cells that are recruited to the injury site by the expression of cytokines in the wound. Cytokine stimulation is also important in converting these undifferentiated cells into the tendon phenotype, characterized by the expression of markers such as tenomodulin and scleraxis. , BMSC can also enter and participate in soft tissue healing. BMSCs delivered on collagen sponges improve healing in animal models of tendon repair, , and stem cells from other origins have been shown to be effective in enhancing repair in several other tendon injury models. Current research is focused on optimizing the isolation and differentiation of stem cells into the tendon phenotype. In the future, it is likely that cells derived from the patient’s own bone marrow, fat, skin, or muscle will be used to augment tendon repair and to populate engineered tendon graft substitutes ( Fig. 34-3 ). My colleagues and I are pursuing one such option in our laboratory now: a decellularized flexor digitorum profundus tendon allograft, reconstituted with stem cells from the patient’s own tissues and lubricated with an engineered surface containing hyaluronic acid (HA) ( Fig. 34-4 ) and lubricin. Such a graft could be used to bridge flexor tendon defects and, finally, to replace like with like.

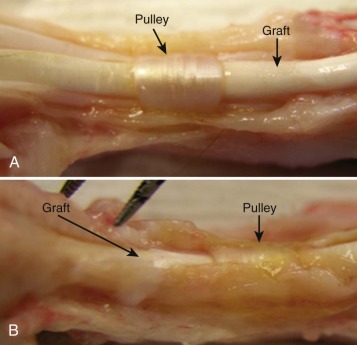
Tendon Lubrication: Hyaluronic Acid and Lubricin
In addition to collagen and structural proteoglycans, such as decorin, the tendon ECM also contains important lubricants for efficient flexor tendon motion (see Fig. 34-1 ). The synovial cells of the flexor tendon sheath secrete HA into the ECM, which may serve as a surface lubricant. HA, a polysaccharide, is found in all vertebrate tissues and body fluids. Various physiologic functions have been assigned to HA, including lubrication, water homeostasis, filtering effects, and regulation of plasma protein distribution. HA is found in increased amounts during the first week after tendon repair. After a tendon is treated with a hyaluronidase solution, which destroys HA, the gliding resistance between the tendon and pulley increases significantly. This suggests that HA on the surface of the flexor tendons may play a role in the surface lubrication of the tendon–pulley system. In vivo results have demonstrated that HA may inhibit the proliferation of rabbit synovial cells, thus preventing cell adhesion between the sheath and the tendon. ,
Recent studies indicate that lubricin, a proteoglycan found in the superficial zone of articular cartilage, may play an important role in preventing cellular adhesions in addition to providing the lubrication necessary for normal joint function. Lubricin was originally isolated from articular cartilage. It has since been identified on the surface of tendons and plays an important role in tendon lubrication. However, lubricin also inhibits cellular adhesion and so has the undesirable effect of inhibiting tissue repair.
The expression of lubricin is modulated by interleukin-1 (IL-1), tumor necrosis factor (TNF-α) and TGF-β. Little else is known about the expression or regulation of lubricin within digital flexor tendons, but its modulation may have a profound effect on the restoration of the flexor surface and the prevention of adhesions after tendon injury and repair ( Fig. 34-5 ) and perhaps as a coating on a tissue-engineered tendon graft or tendon graft substitute, as discussed later.

Engineering the Tendon Surface
The effect of HA on flexor tendon repair has been investigated in animal and clinical studies. Exogenously applied HA may prevent adhesion formation between the flexor tendon and surrounding tissue following tendon repair without affecting tendon healing, although in vivo results have been contradictory. As the half-life of HA in tissue is short, native HA is probably eliminated too rapidly to maintain a long-lasting physical barrier between opposing tissues. Moreover, abrasion during tendon gliding constantly threatens to physically remove HA from the tendon surface. Therefore, extending HA half-life and strengthening HA binding ability on the tendon surface are important to enhancing the clinical effect of exogenously administered HA.
The carbodiimide derivatization, a chemical modification of HA, has been developed recently for clinical use. This modification of HA decreases the water solubility of HA, increases its intermolecular binding strength, and therefore increases tissue residence time. Clinical studies of a proprietary form of this derivatized HA (Seprafilm or Seprafilm II, Genzyme Corp, Cambridge, MA, or Hyaloglide; ACP gel, Fidia Advanced Biopolymers, Abano Terme, Italy), fabricated as a cross-linked sheet to be inserted as a barrier between opposing surfaces where adhesion is undesirable, have shown that it can reduce postsurgical adhesions in gynecologic and abdominal surgery. A variation on this theme, by doing the cross-linking reaction in situ to fix the HA directly to the tendon surface, using collagen as an intermediary (carbodiimide derivatized HA, or cd-HA), has had promising preliminary results in animal studies in vitro and in vivo. The combination of HA and lubricin appears to have an additive effect. Recent work has also shown, though, that although physicochemical and pharmacologic interventions can reduce adhesion formation, in both tendon grafts and tendon repairs, there is a cost in terms of delayed or impaired tendon healing after tendon repair. Newer investigations are considering how to combine adhesion reduction and improved healing through the use of growth factors and stem cells.
Tendon Repair
Over the past 50 years, novel repair techniques have resulted in improved clinical outcomes following flexor tendon surgery. The details of clinical tendon repair are covered in Chapter 35 , but this chapter focuses on the effect of repair constructs on tendon healing and tendon kinematics.
Despite these advances in repair technique, adhesions continue to occur, and results can be less than adequate, particularly when the injury occurs in zone 2, the so called no-man’s land, where the tendon resides within a fibro-osseous pulley system. Critical features related to tendon repair include a strong, minimally reactive repair that maintains strong tendon coaptation while permitting tendon gliding. Two major problems continue to occur within the clinical setting: gapping with rupture at the repair site and adhesion formation within the flexor sheath. Despite attempts at modifying rehabilitation, whether through increased levels of applied load or increased rates, tendon excursion methods have failed to increase early tendon core strength.
Repair Biomechanics
The ideal tendon repair is strong, easy to perform, and does not interfere with either tendon healing or tendon gliding. Current methods are moderately strong and able to withstand the normal forces of light motion. However, some of these constructs, especially those with multiple loops or knots on the anterior tendon surface, also generate high-friction forces with movement and may abrade the pulley surface over time ( Fig. 34-6 ). Newer suture designs have incorporated features such as fewer surface loops, loops on the lateral rather than anterior tendon surfaces, and knots inside the repair rather than on the surface; all these features help reduce friction while having little effect on breaking strength. Newer suture materials, such as FiberWire, a composite suture consisting of a monofilament polyethylene core surrounded by a braided polyester jacket (Arthrex, Naples FL), combine higher breaking strength, so that a smaller-diameter suture can be used, as well as providing low friction. ,


The Effect of Friction on the Results of Tendon Repair
Animal studies over the past decade have shown convincingly that high-friction repairs result in abrasion of the tendon sheath ( Fig. 34-7 ) and adhesion formation, even when factors such as rehabilitation method are optimized. Thus, the goal should be to use a high-strength, low-friction repair construct and a low-friction suture material. Most recently I have been using 3-0 Ethibond and a modified Pennington design, but the recent data noted earlier on FiberWire is certainly intriguing.

Effect on Postoperative Management
Until the mid-1960s, most flexor tendon repairs were immobilized postoperatively for 3 weeks. This policy was based on the research of Mason and Allen, who had shown that canine flexor tendon repairs decreased in tensile strength for 3 weeks postoperatively. Subsequent clinical work by Verdan, Kleinert and Verdan, and Duran and associates showed that human flexor tendon repairs could be safely mobilized with a combination of active extension and passive flexion.
The use of early mobilization after tendon repair has resulted in improved outcomes. In animal models, earlier mobilization results in better final tendon gliding and tensile strength. More recently, the fine details of mobilization have been studied, specifically the effect of timing and the effect of differential motion of the wrist and finger joints on tendon loading and tendon gliding during the healing period. Active motion protocols have also been used, although, interestingly, the clinical results are not reliably better than passive protocols. Moreover, the addition of loading to motion in animal models has been shown to have little effect on the final result in terms of strength and motion. Thus, the available evidence suggests that motion, not load, is the critical factor.
Of course, there must be some load on the tendon if it is going to move; at the very least, the load must be sufficient to overcome the forces of friction. It is for this reason that low-friction repairs are important—they minimize the load needed to initiate movement. Friction, though, is not the only concern. The force needed to overcome joint stiffness and to flex traumatized, edematous tissues must also be considered, as well as the weight of the distal digit itself; often these latter forces far outweigh the frictional ones in magnitude, especially in injured digits. So, the minimum force needed to load the tendon is a combination of the frictional force and the force needed to move the joints and soft tissues. This combination is often called the “work of flexion” of the unloaded digit.
One might imagine that the maximum load that could be applied is the load that represents the breaking strength of the tendon, but that would be incorrect: long before the tendon breaks, it begins to gap, and gapping also increases friction, setting up a vicious cycle that can lead to later rupture. So, really, the upper bound is not breaking strength but the force needed to create a gap, which is usually much less. The difference between the two forces—the unloaded work of flexion and the gapping force—represents the “safe zone” in which rehabilitation can occur ( Fig. 34-8 ). Early on, this safe zone is bounded by strictly mechanical parameters related to the anatomy and biomechanics of the repair. Over time, though, the effects of tendon healing are added in; the general effect is usually to gradually widen the safe zone, enabling the rational use of a graded resistance program as outlined by Groth. The details of such programs are reviewed in Chapter 36 .

Unfortunately, in some cases, early mobilization after tendon repair is not possible by any method. Common examples include situations with complex hand injury, in which motion might jeopardize bone, skin, nerve, or vascular integrity; patients who are uncooperative due to age or mental status; or situations where the tendon repair is deemed to be too tenuous to tolerate mobilization. In such cases, adhesions have been, up to now, inevitable. It is possible, though, that the application of a tissue-engineered, biocompatible adhesion barrier that is porous to nutrients might allow an immobilized tendon to heal without adhesions. We are currently pursuing research to address this issue, using cd-HA and lubricin, linked to collagen, as the proposed barrier, and hope to have an update in time for the next edition of this book!
Summary
In summary, considerable advances have been made in our understanding of tendon healing and both the biology and biomechanics of tendon repair and reconstruction. The “safe zone” concept provides a good framework for thinking about the interaction among friction, repair strength, healing, and loading. Early motion, using the least load possible, is the key to better results, but early motion alone is usually not sufficient to prevent adhesions, without posing an undue risk of repair rupture. Thus, the ideal tendon repair of the future will probably need to include a combination of three features. There will always be a need for better, low-friction repair techniques. Lubricants bound to the tendon surface would further reduce friction, lower the loading requirements, and block adhesions. Cell and cytokine “patches” at the repair site can speed healing and allow a faster widening of the safe zone, which should result in fewer complications. This combination approach would appear to offer the best path toward the ultimate goal of predictable restoration of normal function after tendon injury.
References
- 1. Kelsey JL: [object Object]. In (eds): . New York: Churchill Livingstone, 1997.
- 2. Newmeyer W: History of the . J Hand Surg 2000; 25A: pp. 5-13
- 3. Wilgis EF: Classic papers in hand surgery. [comment]. J Hand Surg Am 2000; 25: pp. 14-18
- 4. Kleinert HE, Serafin D, Kutz JE, and Atasoy E: Reimplantation of amputated digits and hands. Orthop Clin North Am 1973; 4: pp. 957-967
- 5. Harris SB, Harris D, Foster AJ, and Elliot D: The aetiology of acute rupture of flexor tendon repairs in zones 1 and 2 of the fingers during early mobilization. J Hand Surg Br 1999; 24: pp. 275-280
- 6. Kitsis CK, Wade PJ, Krikler SJ, et al: Controlled active motion following primary flexor tendon repair: a prospective study over 9 years. J Hand Surg Br 1998; 23: pp. 344-349
- 7. Elliot D, Moiemen NS, Flemming AF, et al: The rupture rate of acute flexor tendon repairs mobilized by the controlled active motion regimen. J Hand Surg Br 1994; 19: pp. 607-612
- 8. Small JO, Brennen MD, and Colville J: Early active mobilisation following flexor tendon repair in zone 2 [see comments]. J Hand Surg Br 1989; 14: pp. 383-391
- 9. Guo Y, Pili R, and Passaniti A: Regulation of prostate-specific antigen gene expression in LNCaP human prostatic carcinoma cells by growth, dihydrotestosterone, and extracellular matrix. Prostate 1994; 24: pp. 1-10
- 10. Occleston NL, Daniels JT, Tarnuzzer RW, et al: Single exposures to antiproliferatives: long-term effects on ocular fibroblast wound-healing behavior. Invest Ophthalmol Vis Sci 1997; 38: pp. 1998-2007
- 11. Beredjiklian PK: Biologic aspects of flexor tendon laceration and repair. J Bone Joint Surg Am 2003; 85-A: pp. 539-550
- 12. Vogel KG, Keller EJ, Lenhoff RJ, et al: Proteoglycan synthesis by fibroblast cultures initiated from regions of adult bovine tendon subjected to different mechanical forces. Eur J Cell Biol 1986; 41: pp. 102-112
- 13. Rees SG, Flannery CR, Little CB, et al: Catabolism of aggrecan, decorin and biglycan in tendon. Biochem J 2000; 350: pp. 181-188
- 14. Caterson EJ, Nesti LJ, Albert T, et al: Application of mesenchymal stem cells in the regeneration of musculoskeletal tissues. Medgenmed Computer File. MedGenMed 2001; undefined: pp. E1
- 15. Hae Yoon J, Brooks R, Kwan Kim Y, et al: Proteoglycans in chicken gastrocnemius tendons change with exercise. Arch Biochem Biophys 2003; undefined: pp. 279-286
- 16. Kryger GS, Chang AK, Costa M, et al: A comparison of tenocytes and mesenchymal stem cells for use in flexor tendon tissue engineering. J Hand Surg Am 2007; 32: pp. 597-605
- 17. Chen MY, Jeng L, Sun YL, et al: Contraction of collagen gels seeded with tendon cells. Biorheology 2006; 43: pp. 337-345
- 18. Boyer MI, Watson JT, Lou J, et al: Quantitative variation in vascular endothelial growth factor mRNA expression during early flexor tendon healing: an investigation in a canine model. J Orthop Res 2001; 19: pp. 869-872
- 19. Aspenberg P: Stimulation of tendon repair: mechanical loading, GDFs and platelets. A mini-review. Int Orthop 2007; 31: pp. 783-789
- 20. Salo P, Bray R, Seerattan R, et al: Neuropeptides regulate expression of matrix molecule, growth factor and inflammatory mediator mRNA in explants of normal and healing medial collateral ligament. Regul Pept 2007; 142: pp. 1-6
- 21. Tsubone T, Moran SL, Amadio PC, et al: Expression of growth factors in canine flexor tendon after laceration in vivo. Ann Plast Surg 2004; 53: pp. 393-397
- 22. Chan D, and Cole WG: Quantitation of type I and III collagens using electrophoresis of alpha chains and cyanogen bromide peptides. Anal Biochem 1984; 139: pp. 322-328
- 23. Jozsa L, Reffy A, and Balint JB: Polarization and electron microscopic studies on the collagen of intact and ruptured human tendons. Acta Histochem 1984; 74: pp. 209-215
- 24. Kannus P, Jozsa L, Kvist M, et al: The effect of immobilization on myotendinous junction: an ultrastructural, histochemical and immunohistochemical study. Acta Physiol Scand 1992; 144: pp. 387-394
- 25. Herzog M, Lindsay WK, and McCain WG: Effect of beta-aminioproprionitrile on adhesions following digital flexor tendon repair in chickens. Surg Forum 1970; 21: pp. 509-511
- 26. Kapetanos G: The effect of the local corticosteroids on the healing and biomechanical properties of the partially injured tendon. Clin Orthop Relat Res 1982; 163: pp. 170-179
- 27. Kulick MI, Smith S, and Hadler K: Oral ibuprofen: evaluation of its effect on peritendinous adhesions and the breaking strength of a tenorrhaphy. J Hand Surg Am 1986; 11: pp. 110-120
- 28. Blumenkranz MS, Ophir A, Claflin AJ, and Hajek A: Fluorouracil for the treatment of massive periretinal proliferation. Am J Ophthalmol 1982; 94: pp. 458-467
- 29. Blumenkranz M, Hernandez E, Ophir A, and Norton EW: 5-fluorouracil: new applications in complicated retinal detachment for an established antimetabolite. Ophthalmology 1984; 91: pp. 122-130
- 30. Khaw PT, Grierson I, Hitchings RA, and Rice NS: 5-fluorouracil and beyond. Br J Ophthalmol 1991; 75: pp. 577-578
- 31. Khaw PT, Ward S, Porter A, et al: The long-term effects of 5-fluorouracil and sodium butyrate on human Tenon’s fibroblasts. Invest Ophthalmol Vis Sci 1992; 33: pp. 2043-2052
- 32. Khaw PT, Sherwood MB, MacKay SL, et al: Five-minute treatments with fluorouracil, floxuridine, and mitomycin have long-term effects on human Tenon’s capsule fibroblasts. Arch Ophthalmol 1992; 110: pp. 1150-1154
- 33. Khaw PT, Doyle JW, Sherwood MB, et al: Effects of intraoperative 5-fluorouracil or mitomycin C on glaucoma filtration surgery in the rabbit. Ophthalmology 1993; 100: pp. 367-372
- 34. Doyle JW, Sherwood MB, Khaw PT, et al: Intraoperative 5-fluorouracil for filtration surgery in the rabbit. Invest Ophthalmol Vis Sci 1993; 34: pp. 3313-3319
- 35. Moran SL, Ryna CK, Orlando GS, et al: Effects of 5-fluorouracil on flexor tendon repair. J Hand Surg Am 2000; 25: pp. 242-251
- 36. Akali A, Khan U, Khaw PT, and MacGrouther AD: Decrease in adhesion formation by a single application of 5-fluorouracil after flexor tendon injury. Plast Reconstr Surg 1999; 103: pp. 151-158
- 37. Khan U, Occleston NL, Khaw PT, and MacGrouther AD: Single exposures to 5-fluorouracil: a possible mode of targeted therapy to reduce contractile scarring in the injured tendon. Plast Reconstr Surg 1997; 99: pp. 465-471
- 38. Ragoowansi R, Khan U, Brown RA, and MacGrouther DA: Reduction in matrix metalloproteinase production by tendon and synovial fibroblasts after a single exposure to 5-fluorouracil. Br J Plast Surg 2001; 54: pp. 283-287
- 39. Singer AJ, and Clark RAF: Cutaneous wound healing. N Engl J Med 1999; 341: pp. 738-746
- 40. Costa MA, Wu C, Pham BV, et al: Tissue engineering of flexor tendons: Optimization of tenocyte proliferation using growth factor supplementation. Tissue Eng 2006; 12: pp. 1937-1943
- 41. Thomopoulos S, Zeagel M, Das R, et al: PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J Orthop Res 2007; 25: pp. 1358-1368
- 42. Juncosa-Melvin N, Boivin JP, Gooch C, et al: The effects of autologous mesenchymal stem cells on the biomechanics and histology of gel-collagen sponge constructs used for rabbit patellar tendon repair. Tissue Eng 2006; 12: pp. 369-379
- 43. Tang JB, Xu Y, Ding F, and Wang XT: Tendon healing in vitro: promotion of collagen gene expression by βFGF with NF-kappaB gene activation. J Hand Surg 2003; 28: pp. 215-220
- 44. Wang XT, Liu PY, and Tang JB: Tendon healing in vitro: modification of tenocytes with exogenous vascular endothelial growth factor gene increases expression of transforming factor beta but minimally affects expression of collagen genes. J Hand Surg 2005; 30: pp. 222-229
- 45. Aspenberg P, and Forslund C: Enhanced tendon healing with GDF 5 and 6. Acta Orthop Scand 1999; 70: pp. 51-54
- 46. Rickert M, Wang H, Wieloch P, et al: Adenovirus-mediated gene transfer of growth and differentiation factor-5 into tenocytes and the healing rat Achilles tendon. Connect Tissue Res 2005; 46: pp. 175-183
- 47. Dines JS, Weber L, Razzano P, et al: The effect of growth differentiation factor-5–coated sutures on tendon repair in a rat model. J Shoulder Elbow Surg 2007; 16: pp. S215-S221
- 48. Border WA, Lkuda S, Languino LR, et al: Suppression of experimental glomerulonephritis by antiserum against transforming growth factor B1. Nature 1990; 346: pp. 371-374
- 49. Border WA, and Noble NA: Transforming growth factor-B in tissue fibrosis. N Engl J Med 1994; 331: pp. 1286-1292
- 50. Chang J, Thunder R, Most D, et al: Studies in flexor tendon wound healing: neutralizing antibody to TGF-beta1 increases postoperative range of motion. Plast Reconstr Surg 2000; 105: pp. 148-155
- 51. Shah M, Foreman DM, and Ferguson MN: Control of scaring in adult wounds by neutralizing antibody to transforming growth factor-B. Lancet 1992; 339: pp. 213-214
- 52. Wahl SM, Allen JB, and Costa GL: Reversal of acute and chronic synovial inflammation by anti-transforming growth factor-B. J Exp Med 1993; 177: pp. 225-230
- 53. Chang J, Most D, Stelnicki E, et al: Gene expression of transforming growth factor beta-1 in rabbit zone II flexor tendon wound healing: Evidence for dual mechanisms of repair. Plast Reconstr Surg 1997; 100: pp. 937-944
- 54. Natsu-ume T, Nakamura N, Shino K, et al: Temporal and spatial expression of transforming growth factor beta in the healing patellar ligament of the rat. J Orthop Res 1997; 15: pp. 837-843
- 55. Ackermann PW, Ahmed M, and Kreicbergs A: Early nerve regeneration after Achilles tendon rupture—a prerequisite for healing? J Orthop Res 2002; 20: pp. 849-856
- 56. Bring DK, Kreicbergs A, Renström PA, and Ackermann PW: Physical activity modulates nerve plasticity and stimulates repair after Achilles tendon rupture. J Orthop Res 2007; 25: pp. 164-172
- 57. Schäffer M, Beiter T, Becker HD, and Hunt TK: Neuropeptides: mediators of inflammation and tissue repair? Arch Surg 1998; 133: pp. 1107-1116
- 58. Smith CA, Stauber F, Waters C, et al: Transforming growth factor-beta following skeletal muscle strain injury in rats. J Applied Physiol 2007; 102: pp. 755-761
- 59. Ackermann PW, Li J, Lundeberg T, and Kreicbergs A: Neuronal plasticity in relation to nociception and healing of rat Achilles tendon. J Orthop Res 2003; 21: pp. 432-441
- 60. Brain SD: Sensory neuropeptides: their role in inflammation and wound healing. Immunopharmacology 1997; 37: pp. 133-152
- 61. Burssens P, Steyaert A, Forsyth R, et al: Exogenously administered substance P and neutral endopeptidase inhibitors stimulate fibroblast proliferation, angiogenesis and collagen organization during Achilles tendon healing. [Erratum appears in . Foot Ankle Int 2005; 26: pp. 832-839
- 62. Nilsson J, von Euler AM, and Dalsgaard CJ: Stimulation of connective tissue cell growth by substance P and substance k. Nature 1985; 315: pp. 61-63
- 63. Steyaert AE, Burssens PJ, Vercruysse CW, et al: The effects of substance P on the biomechanic properties of ruptures rat Achilles’ tendon. Arch Phys Med Rehabil 2006; 87: pp. 254-258
- 64. Nixon AJ, Goodrich LR, Scimeca MS, et al: Gene therapy in musculoskeletal repair. Ann NY Acad Sci 2007; 1117: pp. 310-327
- 65. Dines JS, Weber L, Razzano P, et al: The effect of growth differentiation factor-5-coated sutures on tendon repair in a rat model. J Shoulder Elbow Surg 2007; 16: pp. S215-221
- 66. White WL: Tendon grafts: A consideration of their source, procurement and suitability. Surg Clin North Am 1960; 40: pp. 403-413
- 67. Boyes JH, and Stark HH: Flexor-tendon grafts in the fingers and thumb. A study of factors influencing results in 1000 cases. J Bone Joint Surg Am 1971; 53: pp. 1332-1342
- 68. Gelberman RH, Seiler JG, Rosenberg AE, et al: Intercalary flexor tendon grafts. A morphological study of intrasynovial and extrasynovial donor tendons. Scand J Plast Reconstr Surg Hand Surg 1992; 26: pp. 257-264
- 69. Abrahamsson SO, Gelberman RH, and Lohmander SL: Variations in cellular proliferation and matrix synthesis in intrasynovial and extrasynovial tendons: an in vitro study in dogs. J Hand Surg Am 1994; 19: pp. 259-265
- 70. Gelberman RH: Flexor tendon physiology: tendon nutrition and cellular activity in injury and repair. Instructional Course Lectures 1985; 34: pp. 351-360
- 71. Duffy FJ, Seiler JG, Gelberman RH, and Hergreuter CA: Growth factors and canine flexor tendon healing: initial studies in uninjured and repair models. J Hand Surg Am 1995; 20: pp. 645-649
- 72. Tsuzaki M, Brigman BE, Yamamoto J, et al: Insulin-like growth factor-I is expressed by avian flexor tendon cells. J Orthop Res 2000; 18: pp. 546-556
- 73. Ngo M, Pahm H, Longaker MT, and Chang J: Differential expression of transforming growth factor-beta receptors in a rabbit zone II flexor tendon wound healing model. Plast Reconstr Surg 2001; 108: pp. 1260-1267
- 74. Docheva D, Hunziker EB, Fässler R, and Brandau O: Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol 2005; 25: pp. 699-705
- 75. Shukunami C, Takimoto A, Oro M, and Hiraki Y: Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol 2006; 298: pp. 234-247
- 76. Fathke C, Wilson L, Hutter J, et al: Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells 2004; 22: pp. 812-822
- 77. Krause DS, Theise ND, Collector MI, et al: Multi-organ, multi lineage engraftment by a single bone marrow -derived stem cell. Cell 2001; 105: pp. 369-377
- 78. Tepper OM, Capla JM, Galiano RD, et al: Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow derived cells. Blood 2005; 105: pp. 1068-1077
- 79. Butler DL, Juncosa-Melvin N, Boivin GP, et al: Functional tissue engineering of tendon repair: a multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res 2008; 26: pp. 1-9
- 80. Cao Y, Liu Y, Liu W, et al: Bridging tendon defects using autologous tenocyte engineered tendon in a hen model. Plast Reconstr Surg 2002; 110: pp. 1280-1289
- 81. Juncosa-Melvin N, Shearn JT, Boivin GP, et al: Effects of mechanical stimulation on the biomechanics and histology of stem cell collagen sponge constructs for rabbit patellar tendon repair. Tissue Eng 2006; 12: pp. 2291-2300
- 82. Liu W, Chen B, Deng D, et al: Repair of tendon defects with dermal fibroblast engineered tendon in a porcine model. Tissue Eng 2006; 12: pp. 775-788
- 83. Zantop T, Gilbert TW, Yoder MC, and Badylak SF: Extracellular matrix scaffolds are repopulated by bone marrow derived cells in a mouse model of Achilles tendon reconstruction. J Orthop Res 2006; 24: pp. 1299-1309
- 84. Zhao C, Sun YL, Amadio PC, et al: Surface treatment of flexor tendon autografts with carbodiimide-derivatized hyaluronic acid. An in vivo canine model. J Bone Joint Surg Am 2006; 88: pp. 2181-2191
- 85. Taguchi M, Sun YL, Zhao C, et al: Lubricin surface modification improves extrasynovial tendon gliding in a canine model in vitro. J Bone Joint Surg Am 2008; 90: pp. 129-135
- 86. Tanaka T, Sun YL, Zhao C, et al: Optimization of surface modifications of extrasynovial tendon to improve its gliding ability in a canine model in vitro. J Orthop Res 2006; 24: pp. 1555-1561
- 87. Sun YL, Yang C, Amadio PC, et al: Reducing friction by chemically modifying the surface of extrasynovial tendon grafts. J Orthop Res 2004; 22: pp. 984-989
- 88. Józsa L, and Kannus P: Histopathological findings in spontaneous tendon ruptures. Scand J Med Sci Sports 1997; 7: pp. 113-118
- 89. Fraser JR, Laurent TC, and Laurent UBG: Hyaluronan: its nature, distribution, functions, and turnover. J Intern Med 1997; 242: pp. 27-33
- 90. Kain CC, Manske PR, Reinsel TE, et al: Reconstruction of the digital pulley in the monkey using biologic and nonbiologic materials. J Orthop Res 1988; 6: pp. 871-877
- 91. Uchiyama S, Amadio PC, Ishikawa J, and An KN: Boundary lubrication between the tendon and the pulley in the finger. J Bone Joint Surg Am 1997; 79: pp. 213-218
- 92. Goldberg RL, and Toole BP: Hyaluronate inhibition of cell proliferation. Arthritis Rheum 1987; 30: pp. 769-778
- 93. Wiig M, Abrahamsson SO, and Lundborg G: Tendon repair–cellular activities in rabbit deep flexor tendons and surrounding synovial sheaths and the effects of hyaluronan: an experimental study in vivo and in vitro. J Hand Surg Am 1997; 22: pp. 818-825
- 94. Jay GD: Characterization of a bovine synovial fluid lubricating factor. I. Chemical, surface activity and lubricating properties. Connect Tissue Res 1992; 28: pp. 71-88
- 95. Marcelino J, Capten JD, Suwairi WM, et al: CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nature Genet 1999; 23: pp. 319-322
- 96. Flannery CR, Hughes CE, Schumacher BL, et al: Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and Is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun 1999; 254: pp. 535-541
- 97. Rees S, Davies JR, Tudor D, et al: Immunolocalisation and expression of proteoglycan 4 (cartilage superficial zone proteoglycan) in tendon. Matrix Biol 2002; 21: pp. 593-602
- 98. Swann DA, Sotman S, Dixon M, and Brooks C: The isolation and partial characterization of the major glycoprotein from articular lubricating fraction from bovine synovial fluid. Biochem J 1977; 161: pp. 473-485
- 99. Jay GD, Tantravahi U, Britt DE, et al: Homology of lubricin and superficial zone protein (SZP): products of megakaryocyte stimulating factor (MSF) gene expression by human synovial fibroblasts and articular chondrocytes localized to chromosome 1q25. J Orthop Res 2001; 19: pp. 677-687
- 100. Jay GD, Britt DE, and Cha CJ: Lubricin is a product of megakaryocyte stimulating factor gene expression by human synovial fibroblasts. [Comment]. J Rheumatol 2000; 27: pp. 594-600
- 101. Sun Y, Berger EJ, Zhao C, et al: Mapping lubricin in canine musculoskeletal tissues. Connect Tissue Res 2006; 47: pp. 215-221
- 102. Schaefer DB, Wendt D, Moretti M, et al: Lubricin reduces cartilage—cartilage integration. Biorheology 2004; 41: pp. 503-508
- 103. Amiel D, Ishizue K, Billings E, et al: Hyaluronan in flexor tendon repair. J Hand Surg 1989; 14A: pp. 837-843
- 104. St Onge R, Weiss C, Denlinger JL, and Balazs EA: A preliminary assessment of Na-hyaluronate injection into “no man’s land” for primary flexor tendon repair. Clin Orthop Relat Res 1980; 146: pp. 269-275
- 105. Meyers SA, Seaber AV, Glisson RR, and Nunley JA: Effect of hyaluronic acid/chondroitin sulfate on healing of full-thickness tendon lacerations in rabbits. J Orthop Res 1989; 7: pp. 683-689
- 106. Hagberg L: Exogenous hyaluronate as an adjunct in the prevention of adhesions after flexor tendon surgery: a controlled clinical trial. J Hand Surg Am 1992; 17: pp. 132-136
- 107. Miller JA, Ferguson RL, Powers DL, et al: Efficacy of hyaluronic acid/nonsteroidal anti-inflammatory drug systems in preventing postsurgical tendon adhesions. J Biomed Mater Res 1997; 38: pp. 25-33
- 108. Gaughan EM, Nixon AJ, Krook LP, et al: Effects of sodium hyaluronate on tendon healing and adhesion formation in horses. Am J Vet Res 1991; 52: pp. 764-773
- 109. Salti NI, Tuel RJ, and Mass DP: Effect of hyaluronic acid on rabbit profundus flexor tendon healing in vitro. J Surg Res 1993; 55: pp. 411-415
- 110. Weiss C: The inhibition of flexor tendon adhesions. Bull Hosp Joint Dis Orthop Inst 1986; 46: pp. 193-194
- 111. Weiss C, Suros JM, Michalow A, et al: The role of Na-hylan in reducing postsurgical tendon adhesions: Part 2. Bull Hosp Joint Dis Orthop Inst 1987; 47: pp. 31-39
- 112. Foland JW, Trotter GW, Powers BE, et al: Effect of sodium hyaluronate in collagenase-induced superficial digital flexor tendinitis in horses. Am J Vet Res 1992; 53: pp. 2371-2376
- 113. Band PA: The chemistry, biology, and medical application of hyaluronan and its derivatives. In Laurent TC (eds): Hyaluronan Derivatives: Chemistry and Clinical Applications. London U.K.: Portland Press, 1998. pp. 33-42
- 114. Momose T, Amadio PC, Sun YL, et al: Surface modification of extrasynovial tendon by chemically modified hyaluronic acid coating. J Biomed Mater Res 2002; 59: pp. 219-224
- 115. Bulpitt P, and Aeschlimann D: New strategy for chemical modification of hyaluronic acid: preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J Biomed Mater Res 1999; 47: pp. 152-169
- 116. Hanthamrongwit M, Reid WH, and Grant MH: Chondroitin-6-sulphate incorporated into collagen gels for the growth of human keratinocytes: the effect of cross-linking agents and diamines. Biomaterials 1996; 17: pp. 775-780
- 117. Kuo JW, Swann DA, and Prestwich GD: Chemical modification of hyaluronic acid by carbodiimides. Bioconjug Chem 1991; 2: pp. 232-241
- 118. Tzianabos AO, Cisneros RL, Gershkovitch J, et al: Effect of surgical adhesion reduction devices on the propagation of experimental intra-abdominal infection. Arch Surg 1999; 134: pp. 1254-1259
- 119. Burns JW, Skinner K, Colt MJ, et al: A hyaluronate based gel for the prevention of postsurgical adhesions: evaluation in two animal species. Fertil Steril 1996; 66: pp. 814-821
- 120. Leach RE, Burns JW, Dawe EJ, et al: Reduction of postsurgical adhesion formation in the rabbit uterine horn model with use of hyaluronate/carboxymethylcellulose gel. Fertil Steril 1998; 69: pp. 415-418
- 121. Brunelli G, Longinotti C, Bertazzo C, et al: Adhesion reduction after knee surgery in a rabbit model by Hyaloglide, a hyaluronan derivative gel. J Orthop Res 2005; 23: pp. 1377-1382
- 122. Zhao C, Sun YL, Amadio PC, et al: Surface treatment of flexor tendon autograft with carbodiimide-derivatized hyaluronic acid. An in vivo canine model. J Bone Joint Surg Am 2006; 88: pp. 2181-2191
- 123. Tanaka T, Zhao C, Sun YL, et al: The effect of carbodiimide-derivatized hyaluronic acid and gelatin surface modification on peroneus longus tendon graft in a short-term canine model in vivo. J Hand Surg Am 2007; 32: pp. 876-881
- 124. Zhao C, Amadio PC, Tanaka T, et al: Short-term assessment of optimal timing for postoperative rehabilitation after flexor digitorum profundus tendon repair in a canine model. J Hand Ther 2005; 18: pp. 322-329
- 125. Cetin A, Dinçer F, Keçik A, and Cetin M: Rehabilitation of flexor tendon injuries by use of a combined regimen of modified Kleinert and modified Duran techniques. Am J Phys Med Rehabil 2001; 80: pp. 721-728
- 126. Helm R: The management of flexor tendon injuries in zone II. Curr Orthop 2002; 16: pp. 434-444
- 127. Elliot D: Primary flexor tendon repair—operative repair, pulley management and rehabilitation. J Hand Surg Br 2002; 27: pp. 507-513
- 128. Golash A, Kay A, Warner JG, et al: Efficacy of ADCON-T/N after primary flexor tendon repair in zone II: a controlled clinical trial. J Hand Surg Br 2003; 28: pp. 113-115
- 129. Miller B, Dodds SD, deMars A, et al: Flexor tendon repairs: the impact of fiberwire on grasping and locking core sutures. J Hand Surg Am 2007; 32: pp. 591-596
- 130. Silfverskiold KL, and Andersson CH: Two new methods of tendon repair: an in vitro evaluation of tensile strength and gap formation. J Hand Surg Am 1993; 18: pp. 58-65
- 131. Aoki M, Manske PR, Pruitt DL, and Larson BJ: Work of flexion after tendon repair with various suture methods. A human cadaveric study. J Hand Surg Br 1995; 20: pp. 310-313
- 132. Zhao C, Amadio PC, Zobitz ME, and An KN: Gliding characteristics of tendon repair in canine flexor digitorum profundus tendons. J Orthop Res 2001; 19: pp. 580-586
- 133. Momose T, Amadio PC, Zhao C, et al: Evaluation of suture techniques with high breaking strength and low gliding resistance. Acta Orthop Scand 2001; 72: pp. 635-641
- 134. Paillard PJ, Amadio PC, Zhao C, et al: Gliding resistance after FDP and FDS tendon repair in zone II: an in vitro study. Acta Orthop Scand 2002; 73: pp. 465-470
- 135. Zhao C, Amadio PC, Paillard PJ, et al: Digital resistance and tendon strength during the first week after flexor digitorum profundus tendon repair in a canine model in vivo. J Bone Joint Surg Am 2004; 86-A: pp. 320-327
- 136. Gelberman RH, Boyer MI, Brodt MD, et al: The effect of gap formation at the repair site on the strength and excursion of intrasynovial flexor tendons. An experimental study on the early stages of tendon-healing in dogs. J Bone J Surg Am 1999; 81: pp. 975-982
- 137. Khan U, Kakar S, Akali A, et al: Modulation of the formation of adhesions during the healing of injured tendons. J Bone Joint Surg Br 2000; 82: pp. 1054-1058
- 138. Boyer MI, Meunier MJ, Lescheid J, et al: The influence of cross-sectional area on the tensile properties of flexor tendons. J Hand Surg Am 2001; 26: pp. 828-832
- 139. Lieber RL, Amiel D, Kaufman KR, et al: Relationship between joint motion and flexor tendon force in the canine forelimb. J Hand Surg 1996; 21A: pp. 957-962
- 140. Lieber RL, Silva MJ, Amiel D, and Gelberman RH: Wrist and digital joint motion produce unique flexor tendon force and excursion in the canine forelimb. J Biomech 1999; 32: pp. 175-181
- 141. Silva M, Brodt MD, Boyer MI, et al: Effects of increased in vivo excursion on digital range of motion and tendon strength following flexor tendon repair. J Orthop Res 1999; 17: pp. 777-783
- 142. McLarney E, Hoffman H, and Wolfe SW: Biomechanical analysis of the cruciate four-strand flexor tendon repair. J Hand Surg Am 1999; 24: pp. 295-301
- 143. Lin GT, An KN, Amadio PC, and Cooney WP: Biomechanical studies of running suture for flexor tendon repair in dogs. J Hand Surg Am 1988; 13: pp. 553-558
- 144. Taras JS, Raphael JS, Marczyk SC, and Bauerle WB: Evaluation of suture caliber in flexor tendon repair. J Hand Surg Am 2001; 26: pp. 1100-1104
- 145. Momose T, Amadio PC, Zhao C, et al: Suture techniques with high breaking strength and low gliding resistance: experiments in the dog flexor digitorum profundus tendon. Acta Orthop Scand 2001; 72: pp. 635-641
- 146. Silva JM, Zhao C, An KN, et al: Gliding resistance and strength of composite sutures in human flexor digitorum profundus tendon repair: an in vitro biomechanical study. J Hand Surg Am 2009; 34: pp. 87-92
- 147. Schuind F, Garcia-Elias M, Cooney WP, and An KN: Flexor tendon forces in vivo measurements. J Hand Surg Am 1992; 17: pp. 291-298
- 148. Momose T, Zhao C, An K, et al. Gliding resistance and breaking strength of suture techniques with knots inside the repair site. 46th Annual Meeting of Orthopaedic Research Society. Orlando, FL, 2000.
- 149. Momose T, Amadio PC, Zhao C, et al: The effect of knot location, suture material, and suture size on the gliding resistance of flexor tendons. J Biomed Mater Res 2000; 53: pp. 806-811
- 150. Zhao C, Amadio PC, Mamose T, et al: Remodeling of the gliding surface after flexor tendon repair in a canine model in vivo. J Orthop Res 2002; 20: pp. 857-862
- 151. Amadio PC, and Amadio PC: Friction of the gliding surface. Implications for tendon surgery and rehabilitation. J Hand Ther 2005; 18: pp. 112-119
- 152. Miller B, Dodds SD, deMars A, et al: Flexor tendon repairs: the impact of fiberwire on grasping and locking core sutures. J Hand Surg Am 2007; 32: pp. 591-596
- 153. Zhao C, Amadio PC, Momose T, et al: Effect of synergistic wrist motion on adhesion formation after repair of partial flexor digitorum profundus tendon lacerations in a canine model in vivo. J Bone Joint Surg 2002; 84-A: pp. 78-84
- 154. Zhao C, Amadio PC, Momose T, et al: The effect of suture technique on adhesion formation after flexor tendon repair for partial lacerations in a canine model. J Trauma 2001; 51: pp. 917-921
- 155. Mason M, and Allen H: The rate of healing of tendons. An experimental study of tensile strength. Ann Surg 1941; 113: pp. 424-459
- 156. Verdan CE: Half a century of flexor-tendon surgery. Current status and changing philosophies. J Bone Joint Surg Am 1972; 54: pp. 472-4791
- 157. Kleinert HE, and Verdan C: Report of the Committee on Tendon Injuries (International Federation of Societies for Surgery of the Hand). J Hand Surg Am 1983; 8: pp. 794-798
- 158. Duran RJ, Houser RG, and Stover MG: Management of flexor tendon lacerations in zone 2 using controlled passive motion. In Hunter JM (eds): Rehabilitation of the Hand. St. Louis, MO: CV Mosby, 1978. pp. 217-224
- 159. Lister GD, Kleinert HE, Kutz JE, and Atasoy E: Primary flexor tendon repair followed by immediate controlled mobilization. J Hand Surg Am 1977; 2: pp. 441-451
- 160. Strickland JW: Flexor tendon surgery. Part 1: Primary flexor tendon repair see comments]. J Hand Surg Br 1989; 14: pp. 261-272
- 161. Silfverskiold KL, and May EJ: Flexor tendon repair in zone II with a new suture technique and an early mobilization program combining passive and active flexion [see comments]. J Hand Surg Am 1994; 19: pp. 53-60
- 162. Woo SL-Y, Gelberman RH, Cobb NG, et al: The importance of controlled passive mobilization in flexor tendon healing. Acta Orthop Scand 1981; 52: pp. 615-622
- 163. Bishop AT, Cooney WP, and Wood MB: Treatment of partial flexor tendon lacerations: the effect of tenorrhaphy and early protected mobilization. J Trauma 1986; 26: pp. 301-312
- 164. Hitchcock TF, Light TR, Bunch WH, et al: The effect of immediate constrained digital motion on the strength of flexor tendon repairs in chickens. J Hand Surg 1987; 12A: pp. 590-595
- 165. Gelberman RH, Woo SL, Amiel D, et al: Influences of flexor sheath continuity and early motion on tendon healing in dogs. J Hand Surg Am 1990; 15: pp. 69-77
- 166. Nessler JP, Amadio PC, Berglund LJ, and An KN: Healing of canine tendon in zones subjected to different mechanical forces. J Hand Surg Br 1992; 17: pp. 561-568
- 167. Zhao CF, Amadio PC, Momose T, et al. The effect of synergistic motion on FDP excursion after tendon repair in a canine model in vivo. 46th Annual Meeting of Orthopaedic Research Society. Orlando, FL 2000.
- 168. Adolfsson L, Söderberg G, Larsson M, and Karlander LE: The effects of a shortened postoperative mobilization programme after flexor tendon repair in zone 2. J Hand Surg Br 1996; 21: pp. 67-71
- 169. Halikis MN, Mankse PR, Kubota H, and Aoki M: Effect of immobilization, immediate mobilization, and delayed mobilization on the resistance to digital flexion using a tendon injury model. J Hand Surg Am 1997; 22: pp. 464-472
- 170. Zhao C, Amadio PC, Paillard P, et al: Digital resistance and tendon strength during the first week after flexor digitorum profundus tendon repair in a canine model in vivo. J Bone Joint Surg Am 2004; 86A: pp. 320-327
- 171. Sántha E, Szarvas J, Szabó L, and Répásy E: [Active movement therapy after flexor tendon suture using a new dynamic control splint][Article in German]. Handchir Mikrochir Plast Chir 1998; 30: pp. 312-316
- 172. Lieber R, Silva MJ, Amiel D, and Gelbern RH: Wrist and digital joint motion produce unique flexor tendon force and excursion in the canine forelimb. J Biomech 1999; 32: pp. 175-181
- 173. Cooney WP, Wiedman K, Malo D, and Wood MB: Management of acute flexor tendon injury in the hand. Instr Course Lect 1985; 34: pp. 373-381
- 174. Saldana MJ, Chow JA, Gerbin P, et al: Further experience in rehabilitation of zone II flexor tendon repair with dynamic traction splinting. Plast Reconstr Surg 1991; 87: pp. 543-546
- 175. Amadio P, Berglund L, and An KN: Effect of Weightbearing Stress on Healing of Biologically Distinct Zones of Canine Flexor Tendon. La Jolla, CA: First World Congress of Biomechanics, 1990.
- 176. Tanaka T, Amadio PC, Zhao C, et al: Gliding resistance versus work of flexion—two methods to assess flexor tendon repair. J Orthop Res 2003; 21: pp. 813-818
- 177. Yang C, Zhao C, Amadio PC, et al: Total and intrasynovial work of flexion of human cadaver flexor digitorum profundus tendons after modified Kessler and MGH repair techniques. J Hand Surg Am 2005; 30: pp. 466-470
- 178. Hotokezaka S, and Manske PR: Differences between locking loops and grasping loops: effects on 2-strand core suture [see comments]. J Hand Surg Am 1997; 22: pp. 995-1003
- 179. Sanders DW, Milne AD, Dobravec A, et al: Cyclic testing of flexor tendon repairs: an in vitro biomechanical study see comments]. J Hand Surg Am 1997; 22: pp. 1004-1010
- 180. Mashadi ZB, and Amis AA: Strength of the suture in the epitenon and within the tendon fibres: development of stronger peripheral suture technique. J Hand Surg Br 1992; 17: pp. 172-175
- 181. Wade PJ, Wetherell RG, and Amis AA: Flexor tendon repair: significant gain in strength from the Halsted peripheral suture technique. J Hand Surg Br 1989; 14: pp. 232-235
- 182. Gelberman RH, Boyer MI, Brodt MD, et al: The effect of gap formation at the repair site on the strength and excursion of intrasynovial flexor tendons. An experimental study on the early stages of tendon-healing in dogs. J Bone Joint Surg Am 1999; 81: pp. 975-982
- 183. Tang JB, Gu YT, Rice K, et al: Evaluation of four methods of flexor tendon repair for postoperative active mobilization. Plast Reconstr Surg 2001; 107: pp. 742-749
- 184. Silva MJ, Boyer MI, Gelberman RH, et al. Repair site gap decreases the strength of repaired canine flexor tendons but does not decrease digital range of motion. in 45th Annual Meeting, Orthopaedic Research Society. 1999B. Anaheim, CA.
- 185. Tanaka T, Amadio PC, Zhao C, et al: Gliding characteristics and gap formation for locking and grasping tendon repairs: a biomechanical study in a human cadaver model. J Hand Surg Am 2004; 29: pp. 6-14
- 186. Zhao C, Moran SL, Cha SS, et al: An analysis of factors associated with failure of tendon repair in the canine model. J Hand Surg Am 2007; 32: pp. 518-525
- 187. Zhao C, Amadio PC, Tanaka T, et al: Effect of gap size on gliding resistance after flexor tendon repair. J Bone Joint Surg Am 2004; 86-A: pp. 2482-2488
- 188. Groth GN: Pyramid of progressive force exercises to the injured flexor tendon. J Hand Ther 2004; 17: pp. 31-42
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


