Acetabular Fractures: Extended Iliofemoral Approach
Craig S. Bartlett
Arthur L. Malkani
Milan K. Sen
David L. Helfet
Indications/Contraindications
Over the past 30 years, advances in surgical approaches, reduction techniques, surgical implants, and preoperative and postoperative evaluation of acetabular fractures have resulted in a dramatic improvement in the outcomes in patients with acetabular fractures. Nevertheless, the management of these injuries continues to be a challenging problem for the orthopedic surgeon, in part, due to the complex anatomy of the pelvis and acetabulum.
The primary goal of operative treatment of acetabular fractures is an accurate reduction of the articular surface such that a congruent hip joint is obtained and normal joint mechanics are restored. This is the primary tenet in the management of all intra-articular fractures, and it is especially important in the weight bearing joints of the lower extremity. In the case of the hip joint, malreduction leads to abnormal loading of the articular cartilage and subsequent painful posttraumatic arthrosis and loss of function.
The indications for operative fixation of acetabular fractures in general include displacement of the articular surface, incongruence of the hip joint, unacceptable roof-arc measurements, incarceration of an intra-articular fragment within the joint, and subluxation of the femoral head. The timing of surgery is dependent upon several factors including the availability of an experienced surgeon; management of associated visceral, skeletal, and soft-tissue injuries; and completion of all imaging studies necessary for preoperative planning. Special situations arise such as in the case of an incarcerated intra-articular fragment, an unreducible femoral-head dislocation, or a femoral head fracture that mandate more urgent intervention to prevent further damage to the articular cartilage or minimize the risk of avascular necrosis of the femoral head. Conversely, a Morel-Lavalle lesion deserves special attention and may delay operative management of the acetabular fracture.
The selection of the proper surgical approach for acetabular exposure may not be straightforward and is largely dependent on the fracture pattern and the experience of the
surgeon. Mayo identified five factors that affect the choice of surgical approach: (a) the fracture pattern; (b) the local soft-tissue conditions; (c) the presence of associated, major, systemic injuries; (d) the age and projected functional status of the patient; and (e) the delay from injury to surgery. Although elaborate, the Letournel-Judet classification system is clinically useful in this regard. Injuries to the pelvic ring must also be considered when determining the surgical approach for an acetabular fracture.
surgeon. Mayo identified five factors that affect the choice of surgical approach: (a) the fracture pattern; (b) the local soft-tissue conditions; (c) the presence of associated, major, systemic injuries; (d) the age and projected functional status of the patient; and (e) the delay from injury to surgery. Although elaborate, the Letournel-Judet classification system is clinically useful in this regard. Injuries to the pelvic ring must also be considered when determining the surgical approach for an acetabular fracture.
Complex fracture patterns often require exposure of both the anterior and posterior columns for adequate visualization and reduction. In these cases, an extensile approach will provide adequate access to the roof of the acetabulum for anatomic restoration of its articular surface. The extended iliofemoral approach was developed by Letournel in 1974. It is one of the three most widely used surgical approaches used to gain access to the acetabulum; the others are the Kocher-Langenbeck and the ilioinguinal approaches.
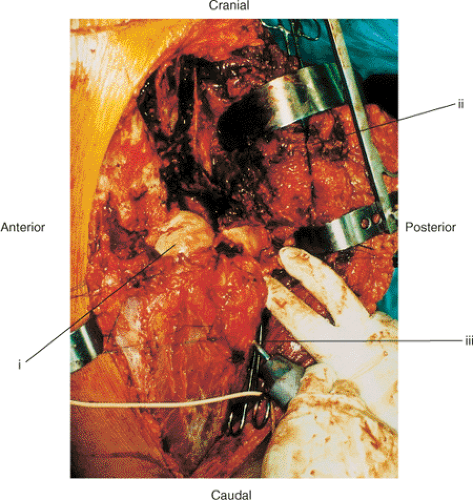
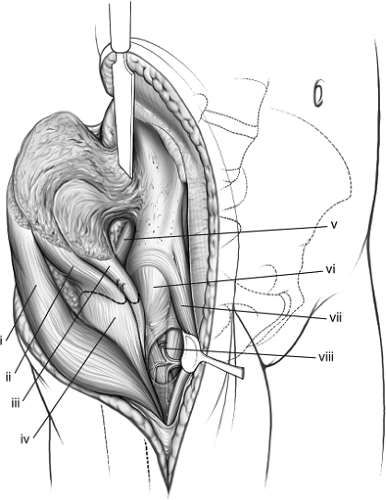
The extended iliofemoral approach allows access to both columns of the acetabulum. It includes the lateral aspect of the iliac wing, the internal iliac fossa, and the retroacetabular surface. It is an anatomic approach that follows an internervous plane in which the muscles innervated by the femoral nerve are reflected anteriorly and the muscles supplied by the superior and inferior gluteal nerves are reflected posteriorly. The posterior flap is mobilized as a unit without damaging its neurovascular bundles (Fig. 43.1).
Specific indications for the extended ilioinguinal approach include (a) high (transtectal) transverse and T-type fracture patterns with involvement of the weight bearing dome (Fig. 43.2); (b) associated anterior column and posterior hemitransverse fractures; (c) associated both-column fractures, with a posterior wall or a comminuted posterior-column lateral-dome involvement (Fig. 43.3) or extension into the sacroiliac joint; (d) transverse or associated fractures where treatment has been delayed. Matta also considered this approach in
certain transverse posterior-wall fractures, such as those involving an extended posterior-wall component where disruption of the retroacetabular surface makes difficult the assessment of reduction completed solely through a Kocher-Langenbeck approach.
certain transverse posterior-wall fractures, such as those involving an extended posterior-wall component where disruption of the retroacetabular surface makes difficult the assessment of reduction completed solely through a Kocher-Langenbeck approach.
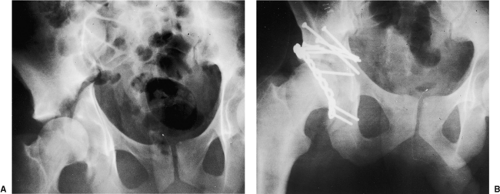 Figure 43.2. A 28-year-old man with right transtectal ischial T-type acetabular fracture. A. Preoperative AP pelvis. B. Postoperative AP pelvis. |
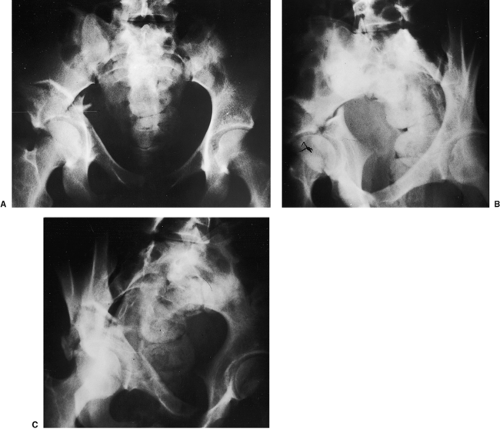 Figure 43.3. An 18-year-old woman with a both-column right acetabular fracture. A. AP pelvis. B. Iliac oblique view. C. Obturator oblique view. |
When there are delays in treatment, the usefulness of approaches such as the ilioinguinal or Kocher-Langenbeck diminishes because of limited joint visualization, organization of the hematoma, increased formation and maturation of callus, and difficulty mobilizing and reducing the fracture lines. Technical problems escalate at approximately 2 weeks after injury with the ability to obtain an anatomic reduction dropping from 75% to 62% of cases by the 3rd week. This is due to the increasingly difficult task of taking down varying amounts of callus, which will progressively interfere with the anatomical reduction of all fractured segments of iliac crest and acetabulum. Even in experienced of hands, late surgical reconstruction of acetabular fractures results in excellent or good outcomes in only 65.5% of cases. Therefore, to improve the likelihood of achieving an anatomic reduction, the extended iliofemoral method is the preferred surgical approach for the most complex acetabular-fracture cases in which surgery has been delayed more than 2 to 3 weeks.
The major technical limitation of the extended iliofemoral exposure is access to the lower portion of the anterior column (Figs. 43.4 and 43.5). Dissection medial to the iliopectineal eminence becomes more difficult where the psoas muscle and iliopectineal fascia block exposure. While a psoas tenotomy can increase visualization, the risk of injury to the femoral artery and nerve must be considered.
There are some relative contraindications to the extended iliofemoral approach. Blunt trauma to the gluteal muscle mass and peritrochanteric region is probably the most common cause for concern. Contusions and abrasions in this area are often associated with the Morel-Lavalle lesion, an area of fluctuance secondary to fatty necrosis and a hematoma that develops under the degloved skin and subcutaneous tissues around the hip. The Morel-Lavalle lesion requires surgical debridement and drainage before internal fixation and is associated with a higher infection rate.
Other relative contraindications to the extended iliofemoral approach include the presence of a closed-head injury, which may lead to massive heterotopic ossification. Extensile approaches are generally avoided in the elderly because of the prolonged operative
time, extensive blood loss, prolonged rehabilitation, and increased risk of infection and heterotopic ossification. Finally, the presence of superior–gluteal vascular injury makes this approach less desirable because ligation of the lateral femoral-circumflex artery during the dissection removes a major source of collateral circulation to the abductor musculature; however, this stance on the procedure remains controversial.
time, extensive blood loss, prolonged rehabilitation, and increased risk of infection and heterotopic ossification. Finally, the presence of superior–gluteal vascular injury makes this approach less desirable because ligation of the lateral femoral-circumflex artery during the dissection removes a major source of collateral circulation to the abductor musculature; however, this stance on the procedure remains controversial.
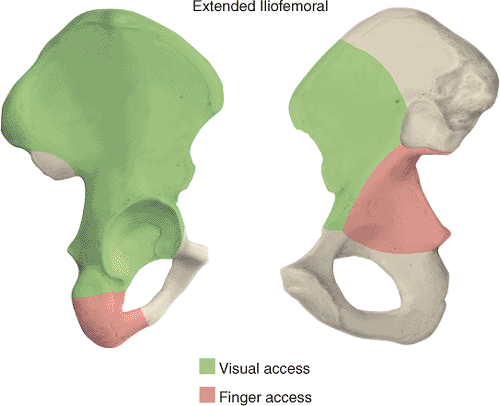 Figure 43.4. Access to the right pelvis via the extended iliofemoral approach. A. Lateral (outer) bony pelvis. B. Medial (inner) bony pelvis. |
Preoperative Planning
The preoperative evaluation begins with a thorough history and physical examination, as well as an appropriate trauma workup, to identify any associated skeletal and visceral injuries. An accurate neurologic examination is mandatory, as the incidence of sciatic nerve injury after acetabular fractures ranges from 12% to 38%.
An accurate diagnosis of the fracture and its subsequent classification can be accomplished with three basic roentgenograms described by Judet et al and include an anteroposterior (AP) view of the pelvis, an iliac oblique view, and an obturator oblique view of the acetabulum. These three roentgenographic views provide sufficient information to allow the surgeon to outline the fracture pattern on a pelvic model as part of the preoperative plan.
Conventional computed tomography (CT) scans with axial views provide additional information about the extent of injury to the acetabulum (Fig. 43.6A), especially identification of posterior-wall fractures, rotation of the columns, the presence of intra-articular fragments or femoral head fractures, and the assessment of articular displacement. Axial CT
scans also can identify associated injuries to the posterior aspect of the pelvis, such as a sacroiliac-joint disruption and sacral fractures. Thin (1 to 2 mm) cuts should be used, along with sagittal and coronal reformatting, to evaluate thoroughly the fracture pattern preoperatively. Advances in imaging software technology have led to the development of three-dimensional CT, which provides an even better understanding of the spatial relation of the fracture pattern relative to the pelvis (see Fig. 43.6B).
scans also can identify associated injuries to the posterior aspect of the pelvis, such as a sacroiliac-joint disruption and sacral fractures. Thin (1 to 2 mm) cuts should be used, along with sagittal and coronal reformatting, to evaluate thoroughly the fracture pattern preoperatively. Advances in imaging software technology have led to the development of three-dimensional CT, which provides an even better understanding of the spatial relation of the fracture pattern relative to the pelvis (see Fig. 43.6B).
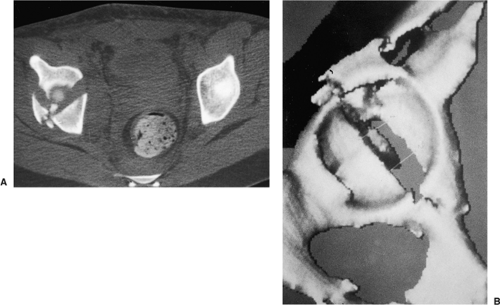 Figure 43.6. CT of pelvis of patient in Figure 43.3. A. Axial view revealing significant dome comminution. B. Three-dimensional reconstruction facilitating perception of configuration. |
Trauma patients, especially those with lower extremity or pelvic injuries, are at extremely high risk for developing deep vein thrombosis (DVT); in some series, the DVT cases are at 60%. We screen all of our acetabular fracture patients for DVT and treat them with compression boots and subcutaneous low-molecular-weight heparin if a delay in surgery is anticipated. Our preferred method of screening is magnetic resonance venography, which we have found to be extremely sensitive and reliable. Patients with an increased risk of DVT or those with documented, preoperative DVT are managed with a vena cava filter and intravenous heparin before surgery.
Surgery
Surgical Anatomy
Three main stages characterize the dissection: (a) elevation of all the gluteal muscles with the tensor fascia lata, (b) division of the external rotators of the hip, and (c) an extended capsulotomy along the lip of the acetabulum. The end result is complete exposure of the outer aspect of the ilium and the whole posterior column inferiorly to the upper part of the ischial tuberosity. Furthermore, the approach may be extended to allow a limited exposure of the internal iliac fossa and the anterior column to the level of the iliopectineal eminence. This allows simultaneous exposure of both columns and permits direct visualization of the reduction and fixation of the anterior and posterior columns (see Fig. 43.4). The articular surface of the acetabulum along with the femoral head may also be visualized if this approach is combined with a surgical dislocation of the hip.
The physician performing an extended iliofemoral approach requires special training and a familiarity with the complex anatomy of the pelvis, particularly the many neurovascular structures that are encountered. Those structures that require identification are listed below.
Sciatic Nerve
The sciatic nerve is at risk during exposure of the posterior column and must be identified, as in the Kocher-Langenbeck approach, along the belly of the quadratus femoris muscle. Traction along the nerve should be minimized by maintaining the hip in extension with the knee flexed at all times.
Lateral Femoral Cutaneous Nerve
The lateral femoral cutaneous nerve is at risk during exposure of the anterior–superior iliac spine. It is also very susceptible to a traction injury during mobilization of the soft tissues. Patients should be warned preoperatively of the significant risk of numbness in the anterolateral thigh after this exposure.
Superior–Gluteal Neurovascular Bundle
The superior–gluteal neurovascular bundle is at risk during exposure of the greater sciatic notch. Therefore, it must be protected from undue traction or penetration by retractors.
Femoral Neurovascular Structures
The medial margin of the extended iliofemoral approach is the iliopsoas muscle and the iliopectineal eminence. Further medial dissection without an ilioinguinal incision places the femoral neurovascular structures at risk.
Pudendal Nerve
The pudendal nerve is at risk as it exits the pelvis through the greater sciatic notch, wraps around the ischial spine, and travels back into the pelvis through the lesser sciatic notch.
Operating Room Preparation
General or spinal anesthesia is administered. We prefer the continuous epidural anesthesia as it provides improved postoperative pain relief. A Foley catheter is placed in the patient’s bladder. The patient is supported on a beanbag and placed in the lateral decubitus position on a radiolucent operating table or fracture table, depending on the surgeon’s preference.
Vascular access in two separate sites with large-bore catheters is important for these lengthy procedures in which significant blood loss is common. The patient’s age and medical condition often dictate placement of an arterial or central line.
We routinely use an intraoperative cell saver to minimize transfusion requirements. This permits recycling of about 20% to 30% of the effective blood loss and is best used when blood loss of more than 2 L is expected.
The hip is kept extended and the knee flexed throughout the procedure to minimize sciatic nerve injury. In addition, intraoperative sciatic-nerve monitoring with spontaneous electromyography (EMG) and somatosensory evoked potentials (SSEP) is used in all cases. The entire pelvis, hip, abdomen, and involved extremity are prepped free, and sterile subdermal electrodes are inserted. The sensory electrodes are inserted adjacent to the common peroneal and posterior tibial nerves and the motor adjacent to the tibialis anterior, peroneus longus, abductor hallucis, and flexor hallucis brevis. The ground is inserted in the heel.
Surgical Approach
The incision is in the form of an inverted “J” (Fig. 43.7) and begins at the posterior–superior iliac spine, extending along the iliac crest toward the anterior–superior iliac spine (ASIS). From here, the distal arm of the incision proceeds along the anterolateral aspect of the thigh for a distance of 15 to 20 cm (Fig. 43.8). The surgeon has a tendency to make this arm more medial than is desired. To avoid this, one should visualize a point 2 cm lateral to the superolateral pole of the patella. With the leg held in neutral rotation, this location is generally in line with the desired incision. Furthermore, a gentle posterior curve may be helpful in obese patients.
The fascial periosteal layer at the iliac crest is identified (Fig. 43.9) and divided sharply along its avascular “white line,” where bleeding will be minimized. Often it is easiest to start in the area of the gluteus medius tubercle where landmarks are more obvious and to progress posteriorly and anteriorly from this point. Posteriorly, the strong fibrous origins of
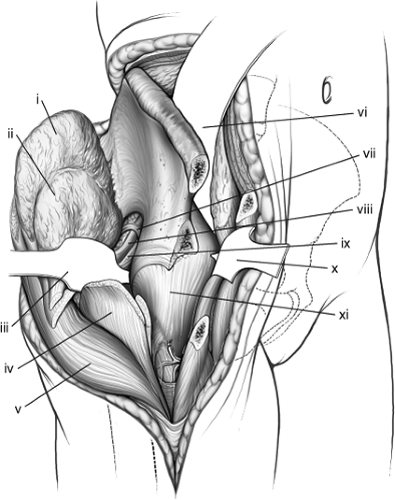
the gluteus maximus should be sharply released from the crista glutei. Depending on the starting location, the tensor fascia-lata muscle and the gluteus medius are subperiosteally released in a stepwise fashion from the outer aspect of the iliac crest (Fig. 43.10). Using an elevator, the musculature along the external surface of the iliac wing is released up to the superior border of the greater sciatic notch and anterosuperior aspect of the hip joint capsule (Fig. 43.11). During this segment of the exposure, care must be taken to identify the superior-gluteal neurovascular bundle, which is at risk as it exits from the notch.
the gluteus maximus should be sharply released from the crista glutei. Depending on the starting location, the tensor fascia-lata muscle and the gluteus medius are subperiosteally released in a stepwise fashion from the outer aspect of the iliac crest (Fig. 43.10). Using an elevator, the musculature along the external surface of the iliac wing is released up to the superior border of the greater sciatic notch and anterosuperior aspect of the hip joint capsule (Fig. 43.11). During this segment of the exposure, care must be taken to identify the superior-gluteal neurovascular bundle, which is at risk as it exits from the notch.
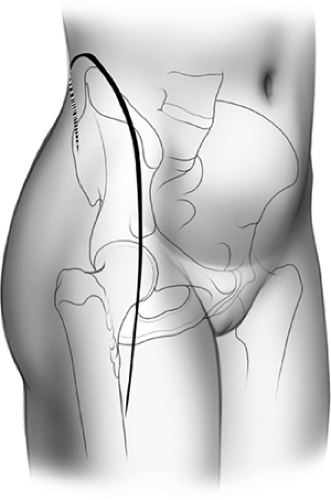 Figure 43.8. Anterolateral view, right side. The inverted-J skin incision with distal extension for the extended iliofemoral approach. |
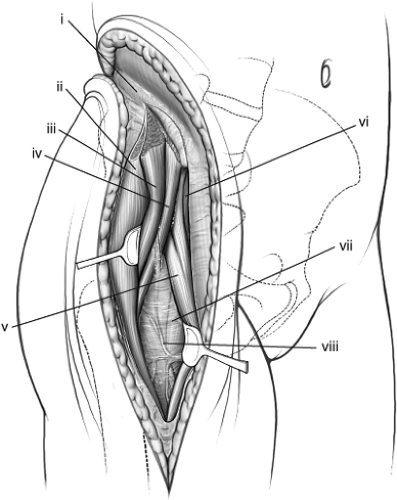
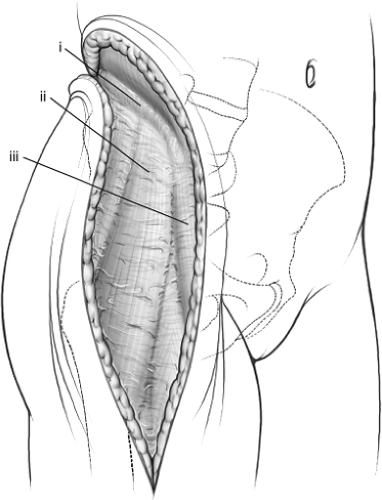 Figure 43.9. Subfascial exposure of right iliac crest and anterior distal limb. (i) Avascular white line. (ii) Fascia covering tensor fascia-lata muscle. (iii) Fascia covering sartorius muscle. |
Attention turns next to the anterior portion of the approach (see Fig. 43.9). The distal limb of the incision is carried over the fascia covering the tensor fascia-lata muscle, and the muscle sheath is entered. The surgeon must stay within the bounds of the sheath, as this will keep the dissection lateral to the lateral femoral-cutaneous nerve, sparing the majority of its branches. It is often helpful to open the sheath from distal to proximal.
Next, the tensor fascia muscle is reflected off its fascia and retracted laterally and upward to expose the floor of the sheath and fascia overlying the rectus femoris muscle (see Fig. 43.10). Small vessels from the superficial circumflex artery are divided and coagulated close to the bone between the superior and inferior spines. Distally, the incision must be long enough to expose the inferior aspect of the muscle belly. This facilitates further release of the gluteal muscles from the crest.
The fascia overlying the rectus muscle is divided longitudinally and horizontally, and its reflected head and direct heads retracted downward and medially to expose a very strong aponeurosis (the “no name” fascia) over the vastus lateralis muscle (see Fig. 43.10). When the rectus is retracted, a constant small vascular pedicle reaching the lateral border of the muscle always requires coagulation. The aponeurosis can be divided longitudinally to expose the ascending branches of the lateral circumflex vessels, which must be isolated and ligated (see Fig. 43.11). Should the upper portion of this exposure be unnecessary, these vessels can occasionally be spared.
Next the thin sheath of the iliopsoas muscle is exposed and longitudinally incised. This allows the use of an elevator to strip the fibers of the psoas from the anterior and inferior aspects of the hip capsule. The exposure of the iliac wing is complete when the reflected head of the rectus femoris is sharply released from its insertion.
The gluteus minimus tendon is identified as it inserts into the anterior edge of the greater trochanter and tagged and transected, leaving a 3- to 5-mm cuff for repair (Figs. 43.11 and 43.12). The gluteus minimus muscle also has extensive attachments to the superior aspect of the hip capsule that may need to be released. Posteriorly and superiorly, the gluteus medius tendon, measuring 15 to 20 mm in length, is also isolated, tagged, and transected, leaving a 3- to 5-mm cuff (see Figs. 43.11




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree