Abstract
Objectives
Survivors of severe traumatic brain injury have a great variety of impairments and participation restrictions. Detailed descriptions of their long-term outcome are critical. We aimed to assess brain injury outcome for subjects with traumatic brain injury in terms of the International classification of functioning, disability and health.
Materials and methods
Four-year follow-up of an inception cohort of adults with severe traumatic brain injury by using face-to-face interviews with patients and proxies.
Results
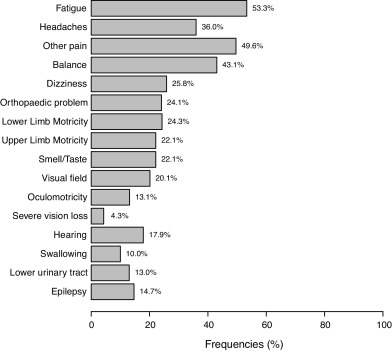
Among 245 survivors at 4 years, 147 were evaluated (80% male, mean age: 32.5 ± 14.2 years at injury); 46 (32%) presented severe disability, 58 (40%) moderate disability, and 40 (28%) good recovery. Most frequent somatic problems were fatigue, headaches, other pain, and balance. One quarter of subjects had motor impairments. Rates of cognitive complaints ranged from 25 to 68%, the most frequent being memory, irritability, slowness and concentration. With the Hospital Anxiety and Depression Scale, 43% had anxiety and 25% depression. Overall, 79% were independent in daily living activities and 40 to 50% needed help for outdoor or organizational activities on the BICRO-39. Most had regular contacts with relatives or close friends but few contacts with colleagues or new acquaintances. Subjects spent little time in productive activities such as working, studying, looking after children or voluntary work. Quality of life on the QOLIBRI scale was associated with disability level ( P < 0.0001).
Conclusion
Management of late brain injury needs to focus on cognitive difficulties, particularly social skills, to enhance patient participation in life.
1
Introduction
Traumatic brain injury (TBI) is an important public health issue, with an overall incidence of 262 hospitalizations/100,000 inhabitants per year in a recent European synthesis , with high frequency among young adults. Severe TBI, although representing about 10% of TBI cases, is responsible for the worse consequences, with 39% early mortality rates and 60% unfavourable outcomes .
A great number of studies have provided data on the outcomes and prognostic factors of severe TBI, but most focused on 6-month or 1-year outcomes . Longitudinal studies with information on late TBI outcome are less frequent . The particularities of the natural history of severe TBI require long-term outcome data. The greater part of recovery takes place within the first year in terms of motor impairments , but changes in cognition or levels of participation are frequently observed at later stages . The late outcome of patients must be described.
TBI outcome is generally understood as mortality or global disability when considered at early stages . However, a specificity of this condition is its multiform nature, with a great variety of long-term consequences . Typical impairments include neurological deficits such as motor, sensory, and cerebellar impairments; muscle tone abnormalities and orthopaedic problems; and various cognitive impairments . Few cohorts have provided detailed descriptions of late impairments after severe TBI , and relative frequencies of each of these are not well known.
As patients strive to resume their pre-injury functioning, late outcome is also involved in consequences of impairments in everyday life. According to the World Health Organization framework of the International Classification of Functioning, Disability and Health (ICF), such consequences include:
- •
limitations in activities that the person can execute;
- •
restrictions in participation, which refers to involvement of the person in real life situations .
Therefore, a comprehensive assessment of TBI outcome should cover a wide range of dimensions, taking into account such activities and participations.
Epidemiological and late outcome data for severe TBI in France comes from several studies. The cohort with TBI of various severity in the Aquitaine region has been followed starting in 1996 and up to 9 years post-injury . The ESPARR longitudinal cohort has been addressing 1- to 5-year outcomes after traffic-related injuries, including TBI, since 2004 . However, the epidemiology is in constant evolution, with a gradual shift from mostly traffic-related injuries in predominantly young males to mostly fall-related injuries in older people in developed countries . Pre-hospital and early care of injuries has also greatly evolved in the past years. Information on post-TBI outcome requires continuous updating. The purpose of the PariS-TBI cohort, which started in 2005–2007, was to renew epidemiological knowledge on TBI and its outcome.
The objective of the present study was to describe the 4-year outcome of this cohort in terms of detailed impairments, activities, participation and quality of life.
2
Patients and methods
2.1
Design of the PariS-TBI study (severe traumatic brain injury in the Parisian area)
The PariS-TBI study was a large prospective inception cohort study undertaken in 2005 in the Île-de-France region (Paris metropolitan area, 12 000 km 2 , 11.6 million inhabitants). Consecutive patients were included by mobile emergency services and firemen brigades of the region over a 22-month period. Criteria for inclusion were patients ≥ 15 years old with severe TBI (lowest Glasgow Coma Scale [GCS] score before hospital admission ≤ 8, in the absence of other causes of coma). A total of 504 patients were included. Main causes of injury were road traffic accident (52%) and falls (34%).
A 1-year outcome assessment, performed by telephone, included measures of overall disability, executive functions and employment . The present report addressed results from the second outcome evaluation conducted 4 years post-injury . Patients and their relatives were contacted by telephone and mail, and a face-to-face interview with a trained neuropsychologist was scheduled. When a direct interview was not possible, patients or their relatives were contacted by telephone to obtain minimal outcome data.
2.2
Patient assessment
The 4-year assessment was designed following the ICF framework of impairments, activities and participations, and used disease-specific validated instruments whenever possible. Overall disability was assessed by the Glasgow Outcome Scale Extended (GOSE) , French version . This widely used scale is based on a structured interview and provides an ordinal classification of disability into 8 categories ranging from death to upper good recovery.
Clinical evaluation and standardized questions assessed the presence of neurological, motor, sensorial, orthopaedic and low urinary tract impairments. Self-reported neuropsychological impairments were provided by questions from the Brain Injury Complaint Questionnaire , a clinician-derived instrument to assess most common cognitive or behavioural changes, with yes/no answers. A short cognitive and behavioural evaluation involved the Neurobehavioural Rating Scale-Revised (NRS-R) . The NRS-R is a 29-item instrument addressing different domains of cognition and behaviour frequently impaired after a TBI, based on a semi-structured interview. For each item, scores range from 1 (absence of dysfunction) to 4 (severe dysfunction); the total score ranges from 29 to 116. The test has been found sensitive to the effect of TBI . Mood impairments were measured by the Hospital Anxiety and Depression Scale (HADS) , which has two subscores, for anxiety and depression, both ranging from 0 to 21 (highest anxiety or depression).
Independence in activities of daily living was measured by the Barthel Index (score ranging from 0 to 100 [full independence], with 5-point increments). To assess real participation in instrumental, social and productive activities, proxy answers to the Brain Injury Community Rehabilitation Outcome-39 scale (BICRO-39) were obtained . This tool, derived from the ICF concepts, has been validated in populations of brain-injured people and translated into French. It includes 8 dimensions (mobility, self-organization, socializing, parent/siblings contact, partner/child contact, productive employment, psychological, personal care), of which the first 6 are presented in this study because psychological difficulties are addressed by the HADS and aspects of personal care by the Barthel Index.
The Quality of life after brain injury scale (QOLIBRI) is a TBI-specific patient-reported measure of quality of life validated in a large multinational TBI population . It includes 37 items measuring 6 dimensions. Four dimensions address the level of satisfaction with cognition (e.g., memory, orientation, decision making), self (e.g., self-image, accomplishments, motivation), daily life and autonomy (in instrumental activities) and social relationships. Two dimensions measure the degree of botheredness with emotions (e.g., feeling of loneliness, anxiety, aggression) and physical problems. It was proposed as a written self-reporting questionnaire; participants were instructed to complete the form at home with a proxy help when necessary and to return it by postal mail.
When only minimal telephone evaluations were possible, these included at least the GOSE assessment, for which face-to-face and telephone assessment are well correlated . General information such as employment status and living place was also obtained.
2.3
Statistical analyses
Data are reported with mean ± SD for quantitative variables and number and percentages for categorical variables. Data were sometimes incomplete, because some participants received shorter telephone interviews and some did not provide answers to all questionnaires. In case of missing data, percentages were based on the number of subjects who answered the given questionnaire.
2.4
Ethical concerns
In accordance with French legislation, patients and their relatives were informed about the initial inclusion in the database, and informed consent of participants or their legal representatives was obtained before late assessments. Approvals from Commissions that enforce research database legislation in France were obtained at each study stage. Approvals from the local Ethical Committee ( Comité de protection des personnes , CPP XI) were obtained before outcome assessments. The study was posted to ClinicalTrials.gov (no. NCT01437683 ).
2
Patients and methods
2.1
Design of the PariS-TBI study (severe traumatic brain injury in the Parisian area)
The PariS-TBI study was a large prospective inception cohort study undertaken in 2005 in the Île-de-France region (Paris metropolitan area, 12 000 km 2 , 11.6 million inhabitants). Consecutive patients were included by mobile emergency services and firemen brigades of the region over a 22-month period. Criteria for inclusion were patients ≥ 15 years old with severe TBI (lowest Glasgow Coma Scale [GCS] score before hospital admission ≤ 8, in the absence of other causes of coma). A total of 504 patients were included. Main causes of injury were road traffic accident (52%) and falls (34%).
A 1-year outcome assessment, performed by telephone, included measures of overall disability, executive functions and employment . The present report addressed results from the second outcome evaluation conducted 4 years post-injury . Patients and their relatives were contacted by telephone and mail, and a face-to-face interview with a trained neuropsychologist was scheduled. When a direct interview was not possible, patients or their relatives were contacted by telephone to obtain minimal outcome data.
2.2
Patient assessment
The 4-year assessment was designed following the ICF framework of impairments, activities and participations, and used disease-specific validated instruments whenever possible. Overall disability was assessed by the Glasgow Outcome Scale Extended (GOSE) , French version . This widely used scale is based on a structured interview and provides an ordinal classification of disability into 8 categories ranging from death to upper good recovery.
Clinical evaluation and standardized questions assessed the presence of neurological, motor, sensorial, orthopaedic and low urinary tract impairments. Self-reported neuropsychological impairments were provided by questions from the Brain Injury Complaint Questionnaire , a clinician-derived instrument to assess most common cognitive or behavioural changes, with yes/no answers. A short cognitive and behavioural evaluation involved the Neurobehavioural Rating Scale-Revised (NRS-R) . The NRS-R is a 29-item instrument addressing different domains of cognition and behaviour frequently impaired after a TBI, based on a semi-structured interview. For each item, scores range from 1 (absence of dysfunction) to 4 (severe dysfunction); the total score ranges from 29 to 116. The test has been found sensitive to the effect of TBI . Mood impairments were measured by the Hospital Anxiety and Depression Scale (HADS) , which has two subscores, for anxiety and depression, both ranging from 0 to 21 (highest anxiety or depression).
Independence in activities of daily living was measured by the Barthel Index (score ranging from 0 to 100 [full independence], with 5-point increments). To assess real participation in instrumental, social and productive activities, proxy answers to the Brain Injury Community Rehabilitation Outcome-39 scale (BICRO-39) were obtained . This tool, derived from the ICF concepts, has been validated in populations of brain-injured people and translated into French. It includes 8 dimensions (mobility, self-organization, socializing, parent/siblings contact, partner/child contact, productive employment, psychological, personal care), of which the first 6 are presented in this study because psychological difficulties are addressed by the HADS and aspects of personal care by the Barthel Index.
The Quality of life after brain injury scale (QOLIBRI) is a TBI-specific patient-reported measure of quality of life validated in a large multinational TBI population . It includes 37 items measuring 6 dimensions. Four dimensions address the level of satisfaction with cognition (e.g., memory, orientation, decision making), self (e.g., self-image, accomplishments, motivation), daily life and autonomy (in instrumental activities) and social relationships. Two dimensions measure the degree of botheredness with emotions (e.g., feeling of loneliness, anxiety, aggression) and physical problems. It was proposed as a written self-reporting questionnaire; participants were instructed to complete the form at home with a proxy help when necessary and to return it by postal mail.
When only minimal telephone evaluations were possible, these included at least the GOSE assessment, for which face-to-face and telephone assessment are well correlated . General information such as employment status and living place was also obtained.
2.3
Statistical analyses
Data are reported with mean ± SD for quantitative variables and number and percentages for categorical variables. Data were sometimes incomplete, because some participants received shorter telephone interviews and some did not provide answers to all questionnaires. In case of missing data, percentages were based on the number of subjects who answered the given questionnaire.
2.4
Ethical concerns
In accordance with French legislation, patients and their relatives were informed about the initial inclusion in the database, and informed consent of participants or their legal representatives was obtained before late assessments. Approvals from Commissions that enforce research database legislation in France were obtained at each study stage. Approvals from the local Ethical Committee ( Comité de protection des personnes , CPP XI) were obtained before outcome assessments. The study was posted to ClinicalTrials.gov (no. NCT01437683 ).
3
Results
3.1
Population characteristics
Among 245 survivors, 147 underwent the 4-year assessment (complete interview or minimal telephone assessment); 62 were lost-to-follow-up, and 36 refused to participate. Mean delay from injury to evaluation was 50.9 ± 6.4 months. Most participants were home-dwellers; 5 were in long-term care facilities. As previously reported , non participants and participants did not differ in age, gender, and TBI severity, but non participants more frequently had a history of pre-injury alcohol abuse or unemployment. Among the 147 subjects included, 117 (80%) were male, and mean age at injury was 32.5 ± 14.2 years (range: 15–81). Mean initial GCS score was 5.9 ± 2.0, and mean time to follow was 13.0 ± 12.3 days.
3.2
Global outcome and impairments
On the GOSE scale ( n = 145), 15 subjects (10%) had lower-level severe disability, 31 (21%) upper-level severe disability, 33 (22%) lower-level moderate disability, 25 (17%) upper-level moderate disability, 33 (22%) lower-level good recovery, and 10 (7%) upper-level good recovery. One person had a tracheostomy, and one had a gastrostomy.
Rates of somatic and neurological impairments are in Fig. 1 ; 128 subjects (83.7%) presented at least one of the impairments. Most frequent problems were fatigue, headaches, other pain, and balance difficulties. Nearly one-quarter of the subjects presented upper-limb or lower-limb motor problems. Most frequent cognitive complaints were memory problems and irritability ( Fig. 2 ). Rates of cognitive complaints ranged from 25% to 68%. Mean HADS scores were 7.2 ± 4.5 for anxiety and 5.2 ± 4.5 for depression ( n = 118). Using the previously defined 8-point cut-off score for these subscales , 51 subjects (43%) had an anxiety disorder and 29 (25%) had depression.