Acute and Chronic Post-traumatic Osteomyelitis
R. Schnettler
Diagnosis of Acute Osteomyelitis (Early Infection)
NOTE
Early diagnosis of an acute infection depends on clinical signs and regular wound assessment.
NOTE
Clinical suspicion of an early infection after internal fracture fixation or operative joint replacement constitutes an urgent, nondeferable indication for operative revision.
The main clinical symptoms of acute osteomyelitis are the basis of early diagnosis and consist of:
• Redness
• Swelling
• Pain
• Local warmth
Laboratory tests showing continued elevation or renewed rise in inflammatory markers—leukocyte count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP)—indicate the presence of an early infection.
Preoperative Preparations
• Radiographs
• Laboratory tests:
– ESR
– CRP
– leukocyte count
– differential blood count
• Smear
• Blood culture if necessary
• Informed consent:
– explanation of the entire treatment concept, as well as the risk of
– recurrence
– amputation
– cancer
Emergency treatment in case of:
• Bacteremia
• Septic shock
• Empyema of the joint
Surgical Procedure
Surgical intervention by primary debridement of the bone and soft tissues is the first step in treating osteomyelitis.
This requires not only basic surgical equipment (Fig. 5.1), but also special instruments (Fig. 5.2). The basic equipment for septic surgery consists of a scalpel and bone forceps (rongeur, Stille-Luer bone rongeur) and, above all, chisels and curettes of different sizes and shapes.
Self-cooling rose-head burrs and bone shavers of different sizes, as well as pulsed jet lavage, complement the basic equipment.

Fig. 5.1 Basic instruments

Fig. 5.2 Special instruments
Primary Interventions on Bones and Soft Tissues
Intraoperative filling of a fistulous tract by injecting indigo carmine or methyl blue through a button cannula facilitates dissection (Figs. 5.3, 5.4, 5.5, 5.6, 5.7, 5.8, 5.9).
Always take biopsies for histologic examination. Differential diagnoses include: Ewing sarcoma, squamouscell carcinoma, and plasma-cell osteomyelitis (Figs. 5.10, 5.11, 5.12, 5.13, 5.14, 5.15, 5.16).
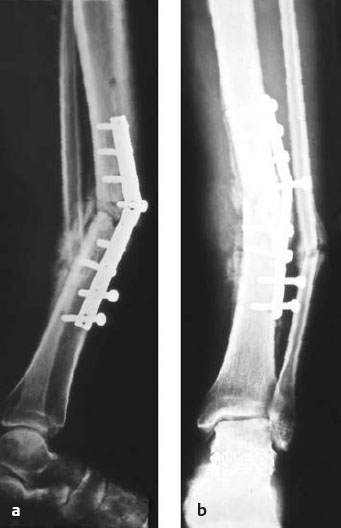
Fig. 5.3a, b Post-traumatic osteomyelitis with sequestration of the middle third of the tibia following plate osteosynthesis.

Fig. 5.4 The surgical approach to a bone infection is the direct approach, for example, by removing a fistula. All unhealthy soft-tissue components are also excised.

Fig. 5.5 After removing the plate, the source of infection is generously excised.

Fig. 5.6 Careful dissection of blood vessels and nerves and documentation in the operation report. A flap was later connected by means of microvascular techniques.

Fig. 5.7 The medullary cavity is opened to provide a complete view of the infection site. For this purpose a narrow, longitudinal groove is made in the bone. The continuity of the long bone should be maintained if at all possible.

Fig. 5.8 Radical osseous debridement begins with removal of all sequestrae.

Fig. 5.9 It is essential to take an intraoperative smear for identification of the pathogen(s) and to perform an antibiogram. Additional tissue samples are taken for microbiologic and histologic examination.
Arrest the blood supply with a tourniquet to obtain a clear view for dissection. Before finishing the operation, release the tourniquet to control bone perfusion. Check vital bone for display of punctate bleeding; if this is not seen, further debridement is necessary.
Antibiotic chains should always be placed at the deepest point in the medullary cavity to ensure an adequate antibiotic level.
Use an overflow drain; otherwise the antibiotic will be suctioned off. Fracture stabilization is maintained with external fixation to avoid the risk of reinfection when using plates or nails.

Fig. 5.10 The medullary cavity is radically debrided using special instruments. Necrotic, infected osseous material is completely removed.

Fig. 5.11a-c Using a rongeur and Stille-Luer bone rongeur (a), water-cooled shaver (b), and medullary-cavity burr (c) facilitates complete debridement of the medullary cavity in both a proximal and distal direction.

Fig. 5.12 For the final removal of tissue debris and reduction of the number of pathogens, the medullary cavity and surrounding soft tissue are irrigated with pulsed jet lavage.

Fig. 5.13 Placement of a local antibiotic chain ensures a highly effective local tissue level of antibiotic.

Fig. 5.14 Intraoperative site with gentamicin-PMMA chain in place.

Fig. 5.15 Postoperative radiographic control following implantation of a gentamicin-PMMA chain.

Fig. 5.16a, b Wound closure must always be without tension. This is achieved either with a primary skin suture (a) or with the aid of poly-urethane foam or a flap (b). Wound secretion is removed with an overflow drain.
Therapeutic Concept in Acute Osteomyelitis (Early Infection)
The complex problems encountered in acute and chronic osteomyelitis can generally only be treated gradually. Only bone consolidation and reconstruction of the surrounding soft tissues can restore the function of the affected extremity. Early surgical revision is unconditionally indicated at the first appearance of clinical signs of a disturbance in wound healing and beginning infection or postoperative hematoma formation. Only then will it be possible to maintain the internal fracture fixation and effectively combat the infection. Stability is of great importance for both fracture healing and removal of the focus of infection; stability delays the spread of infection (Burri 1979, Klemm 1979, Klemm & Schnettler 1992, Klemm 1993, Schnettler 1997).
NOTE
Early intervention within 2 weeks after the primary operation with signs of acute osteomyelitis can often prevent chronic bone infections.
ERRORS AND RISKS
If this is neglected, a chronic infection of bone and soft tissue can develop (Fig. 5.17). (See also p. 112, Diagnosis of Chronic Osteomyelitis)

Fig. 5.17a-c Chronic infection of bone and soft tissues.
Early Infection of Plate Osteosynthesis
Early surgical intervention combined with local antibiotic treatment has proven useful in the treatment of early infection after internal plate fixation.
Operative revision is a complicated procedure and requires a high level of responsibility and experience. The cause of the infection must be identified and removed. Such operations must always be extensive and not be limited to simply opening an infected hematoma.
NOTE
Do not take a smear of pus, as this is often sterile.
Operative revision is always performed in a series of steps, including the sequential removal of biopsies for bacteriologic examination (Figs. 5.18, 5.19, 5.20, 5.21, 5.22).
Pathogens can often be identified with the polymerase chain reaction (PCR), even when smears have produced no results. However, PCR is very susceptible to contamination, which can lead to false-positive results.

Fig. 5.18a, b Illustration of treatment of an early infection after plate osteosynthesis with infected hematoma (a) by removal of the hematoma, debridement, and placement of a gentamicin-PMMA chain above the plate and a drain without suction (b).

Fig. 5.19 Clinical picture of early infection of a plate osteosynthesis of the humerus with wound drainage 6 days postoperative.

Fig. 5.20 Open the full length of the operative wound, remove all suture material, and take a smear and a biopsy for microbiology and histology.
NOTE
Now is the time to administer antibiotics.

Fig. 5.21 Thorough debridement with complete removal of loose bone fragments and avital tissue.
NOTE
If the internal fixation is stable, it can remain in situ. If unstable, an external fixator must be attached before removing the plate to avoid gross dislocations.

Fig. 5.22 Place gentamicin-PMMA chain in a single or double row above the plate (Septopal).
First Step: Clean Out the Infection
Remove the infected hematoma (jet lavage [see also Fig. 5.12], brush), test the stability of the implanted material (each individual screw, see also Fig. 5.20), and test the viability of all tissues (punctate bleeding from bones and tendons, vital color, and consistency of tissues) (Figs. 5.23, 5.24).

Fig. 5.23a, b An overflow drain (without vacuum) leaves the wound through the skin (a). Place a resorbable antibiotic carrier in any of the recesses, if necessary. Take a control radiograph with the gentamicin-PMMA chain in place (b).
NOTE
The gentamicin-PMMA (polymethylmethacrylate) chain is left in place until the fracture fixation devices (metal) have been removed.

Fig. 5.24 Only the skin should be sutured if an infection exists.
Second Step: Bone Consolidation (Figs. 5.25, 5.26):

Fig. 5.25 Open the operation wound to its full extent. Remove the gentamicin-PMMA chain, the screws, and the plates. Take a smear and biopsies for microbiology and histology. Curette the plate bed and the screw canals. Irrigate the wound cavity (with jet lavage if necessary). Place a resorbable antibiotic fleece in the plate bed if necessary. Drain without vacuum (overflow drain). Suture the skin.

Fig. 5.26a, b Bone consolidation without infection.
NOTE
Undisturbed healing with complete and lasting eradication of the infection cannot be expected if avital fragments remain under the plate as a result of disturbed periosteal perfusion.
If this is the case, proceed according to the procedure described in Therapeutic Concept in Chronic Osteomyelitis with Defects up to 4 cm, page 115.
Early Infection after Intramedullary Nailing
The treatment strategy for early infection after intramedullary nailing depends on the implant used. Intramedullary nails that have not been reamed should be removed, whereas reamed intramedullary nails should be left in place. Both procedures, as already described for early infection of plate osteosynthesis, involve opening all operative wounds and thorough debridement. This includes opening the proximal and distal incisions for the intramedullary nail.
Nonreamed Intramedullary Nails
Nonreamed intramedullary nails which have become infected early should be removed (Figs. 5.27, 5.28, 5.29, 5.30, 5.31, 5.32).

Fig. 5.27 After removal of the nail, debridement of the medullary cavity by drilling with a burr 1-2 mm larger than the diameter of the intramedullary nail. Jet lavage of the medullary cavity.

Fig. 5.28 Placement with an applicator of a gentamicin-PMMA chain of appropriate length into the medullary channel.
NOTE
Now is the time to administer an antibiotic.

Fig. 5.29a, b The end of the gentamicin-PMMA chain should always be placed at the deepest point in the medullary canal to ensure that the antibiotic reaches the entire length of the canal.

Fig. 5.30a-c The end of the gentamicin-PMMA chain is left outside of the surgical wound. An overflow drain in placed in the proximal medullary canal. Reosteosynthesis is performed either by means of a reamed intramedullary nail (femur or tibia) or with external fixation (tibia).
NOTE
Reosteosynthesis only after two smears have revealed no pathogens from, for example, irrigation fluid.

Fig. 5.31 Begin gradually removing the chain from the 5th day until complete removal is achieved on the 10th postoperative day, or remove the entire chain on the 10th postoperative day.

Fig. 5.32 Gentamicin-PMMA chain with granulation tissue (20 days in situ).
ERRORS AND RISKS
The beads can become encased in granulation tissue if the chain is removed too late. In this case the chain cannot be pulled out or it tears.
As an alternative to local antibiotic treatment, suction/ irrigation drainage, which has been used for the past four decades, can be applied after removing the intramedullary nail and minimal reaming. This procedure provides mechanical cleaning of the infected region, which is decisive. Not only debris, but also bacteria should be washed away. Prerequisite for the success of the procedure is correct placement of the draining tubes (Fig. 5.33).

Fig. 5.33a, b
a Place the inflow drainage tube at the deepest point (distal) in the medullary canal and the out-flow drain in the proximal medullary canal. Irrigate daily with approximately 3 I.
b Clinical situs
After 3 days, both ends of the drains are attached to Redon bottles.
Smears are taken from the irrigation fluid, but the drains remain in place until the bacteriologic result is available.
NOTE
A second-generation cephalosporin is administered after taking a smear. When pathogens have been identified, appropriate antibiotics are given. Do not overtreat with reserve antibiotics!
If no pathogens have been identified, the drains are removed after 48 hours.
If pathogens have been identified, the suction/irrigation drainage is continued.
Reamed Intramedullary Nails
Reamed intramedullary nails which have become infected early should be left in place. The fundamental operative procedures concerning opening operation wounds and debridement are no different from those of nonreamed intramedullary nails.
First Step: Clean Out the Infection
The status of the infection and the effectiveness of the first operation can be evaluated in the second intervention 24-48 hours later by means of the following procedures:
• Open all wounds
• Remove the gentamicin-PMMA stick
• Irrigate the interior of the intramedullary nail again (with jet lavage if necessary) (Fig. 5.34).
• Take a smear of the irrigation fluid (with biopsies if necessary)
• Clean and reimplant the gentamicin stick (Fig. 5.35)
• Remove the stick 10 days later without anesthesia (Fig. 5.36)

Fig. 5.34 Irrigate the inside of the intramedullary nail with jet lavage if necessary. Take a smear of the irrigation fluid.

Fig. 5.35 Push a gentamicin-PMMA stick (patient-specific production by Biomet, Berlin, Germany) into the middle of the intramedullary nail. The end of the stick should protrude through the skin. Place an overflow drain in the proximal end of the nail.
NOTE
Planned operative revision is obligatory after 24-28 hours in this procedure.
Next Step: Bone Consolidation
As an alternative to the above procedure, the irrigation-vacuum drainage system can be used as described when the intramedullary nail is left in place.

Fig. 5.36 Remove the nail and the locking pins. Carefully ream the medullary cavity. Irrigate again, with jet lavage if necessary. Take a smear from the irrigation fluid and biopsies, if necessary. Insert a gentamicin-PMMA stick into the medullary canal. Remove the stick 10 days later without anesthesia.
NOTE
Open wound treatment or combining local antibiotics with a suction/irrigation drainage system is contraindicated. This leads to loss of an effective antibiotic concentration and the development of resistant pathogens.
Diagnosis of Chronic Osteomyelitis (Late Infection)
Take a thorough medical history including all injuries, operations, hospital stays, and previously identified pathogens.
Physical examination:
• General signs of inflammation:
– redness
– swelling
– pain
– hyperemia
• Local findings:
– fistula
– secretion
– instability of bone
– loosening of fracture fixation devices / prostheses
• Laboratory values:
– ESR
– CRP
– blood count
• Preoperative smear of the fistula
• Imaging diagnosis:
– standard films
– computed tomography (CT) if necessary
– magnetic resonance imaging (MRI) if necessary
– fill fistulous tract with a contrast agent (fistulography) if necessary
• Bone scan (see Chapter 3, Diagnosis of Bone Inflammations with Nuclear Medicine Techniques, p. 54)
NOTE
• Osteomyelitis which appears to have healed can recur even after several years.
• Therefore, it is absolutely necessary to carefully review the previous medical history.
• A secreting fistula is the main symptom of chronic osteomyelitis. General signs of in-flammation are of secondary importance.
• Laboratory parameters are often normal with chronic osteomyelitis. Smears from the fistulous sinus often reveal no pathogens.
ERRORS AND RISKS
It is imperative to plan and perform intraoperative biopsies of the fistula for histologic examination to exclude a carcinoma.
Therapeutic Concept in Chronic Osteomyelitis (Late Infection)
Late Infection after Plate Osteosynthesis
The following procedures are recommended for late infection after plate osteosynthesis with bone consolidation:
• Remove the plate and the screws (Fig. 5.37)
• Take smears and biopsies (for microbiologic and histologic examination)
• Perform radical debridement of the plate bed and screw holes
• Irrigate (with jet lavage, if necessary)
• Apply local antibiotics (resorbable antibiotic carriers, gentamicin-PMMA chain)
• Insert an overflow drain
• Suture the skin
NOTE
If there is a late infection after intramedullary nailing or plate osteosynthesis without bone consolidation, removal of all avital bone and the implant is essential to prevent nonunion (see Fig. 5.17a-c).
Implant a gentamicin-PMMA chain in the defect.
Apply local antibiotics and a spacer.
The further operative procedure depends on the size of the defect (pp. 115–125).

Fig. 5.37 Remove all avital bone particles and all implants.
Late Infection after Intramedullary Nailing
Late infection after intramedullary nailing with bone consolidation is a good indication for short-term local antibiotics (gentamicin-PMMA chain, Septopal).
After removing the intramedullary nail it is very important to carefully ream the medullary canal with a bone-marrow burr 1–2 mm larger than the circumference of the removed nail (gentle debridement). This releases marginal lamellar intramural sequestra, which can then be washed out of the medullary cavity with intensive irrigation (jet lavage, if necessary) (Figs. 5.38 and 5.39).

Fig. 5.38 Diagram of lamellar sequester in the medullary cavity.

Fig. 5.39 Lamellar sequester
NOTE
Lamellar, intramural sequestra are avital and therefore constitute the starting point for recurrent bouts of infection.
NOTE
This is a typical late complication following previous infection after intramedullary nailing.
The operative procedure is exactly the same as that described in the section Early Infection after Intramedullary Nailing: Nonreamed Intramedullary Nails, p. 107).
Encapsulated Phlegmons of Medullary Cavities
These are encapsulated foci of infection that are completely surrounded by bone without local signs of inflammation. Pain at rest during the night in the affected extremity and a medical history of intramedullary nailing are indicators of this type of infection. Radiographs often reveal an osteolytic focus. A bone scan is generally positive (Figs. 5.40, 5.41, 5.42).
• Localize the encapsulated bone abscess with exploratory drill holes
• Pus often escapes under pressure

Fig. 5.40 Pus from the bore hole.

Fig. 5.41 Oval trepanation. Radical debridement.

Fig. 5.42 Septopal chain
• Perform ovular trepanation
• Take smears and biopsies
• Perform radical debridement
• Irrigate (jet lavage if necessary)
• Implant a gentamicin-PMMA chain in the cavity
• Place an overflow drain
Therapeutic Concept in Chronic Osteomyelitis with Defects up to 4 cm
First Step: Clean Out the Infection
1. In the bone:
• Remove metal
• Perform radical debridement and sequestrotomy
• Take smears and biopsies
• Perform stable reosteosynthesis (change of procedure)
• Reduce the number of pathogens with pulsed jet lavage
• Apply local antibiotics (gentamicin-PMMA chain, Septopal) or
• Perform suction/irrigation drainage in exceptional cases
2. In the soft tissues:
• Perform radical debridement
• Close the skin only temporarily (possibly as a vacuum seal)
• Perform serial revisions
NOTE
Only give an antibiotic after a smear has been taken.
Second Step: Reconstruction
1. Of the bone (after 4-6 weeks)
• Perform a revision
• Remove the local antibiotic carrier (Septopal)
• Irrigate intraoperatively (possibly with jet lavage)
• Take smears and biopsies
• Perform autogenous bone grafting
• Perform reconstruction with allografts
• Fill in the defect with iliac-crest bone chips
• Use resorbable bone-replacement materials, possibly mixed with antibiotics
• Use resorbable bone matrix mixed with antibiotics
2. In the soft tissues
Perform the earliest possible definitive skin closure with a
There are different ways to cover a bone and soft tissue defect (Figs. 5.43, 5.44, 5.45, 5.46, 5.47, 5.48, 5.49, 5.50, 5.51, 5.52, 5.53, 5.54, 5.55).

Fig. 5.43 The bone defect is exposed 4 weeks later at the earliest, the local antibiotic is removed, and intraoperative smears are taken. Four weeks earlier radical debridement was performed with administration of a local antibiotic and reconstruction of the soft-tissue defect with a parascapular flap.

Fig. 5.44 The bone defect can be filled in loosely with autogenic chips of trabecular bone (possibly mixed with antibiotics). Here: harvesting from the posterior iliac crest. The high osteogenetic potency of autogenous trabecular bone generally achieves timely bone consolidation.

Fig. 5.45 If there are no autogenous trabecular bone chips available, cryoconserved or heat-stabilized trabecular bone can be used.
NOTE
Legally required laboratory tests must be performed.

Fig. 5.46 Bridging the defect with a tricortical bone graft from the iliac crest provides additional stability.

Fig. 5.47 A 54-year-old female with post-traumatic osteomyelitis after insufficient primary osteosynthesis.

Fig. 5.48 Debridement required. Smear taken and irrigation with pulsed jet lavage after metal removal.
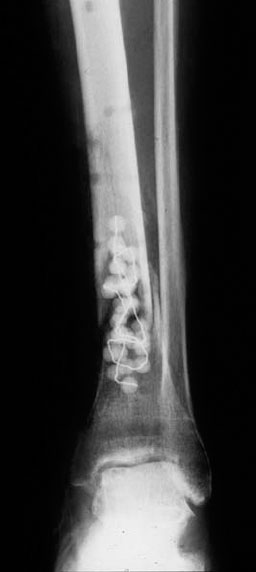
Fig. 5.49 Radical debridement of the bone and soft tissues and local implantation of gentamicin-PMMA chain. Temporary wound closure with polyurethane foam and overflow drain. Temporary immobilization in a lower-leg cast and early secondary soft-tissue coverage with microvascular attachment of a parascapular flap.

Fig. 5.50 After 4-6 weeks lift the flap across from the vascular anastomosis and remove the gentamicin-PMMA chain.

Fig. 5.51 Early secondary coverage of the soft-tissue defect with microvascular attachment of a parascapular flap. Press-fit implantation of a tricortical bone graft from the iliac crest.

Fig. 5.52 Additional stabilization with external fixation.

Fig. 5.53 Three months after early secondary flap transplant—soft tissues free of infection.
NOTE
Daily fixateur-pin care necessary.

Fig. 5.54a,b One year later there is bone consolidation of the formerly infected nonunion, which is stable on weight bearing. The infection has healed.

Fig. 5.55 Clinical picture of healed area.
Therapeutic Concept in Chronic Osteomyelitis with a Defect Larger than 4 cm
First Step
Clean the wound as previously instructed.
Second Step
Perform reconstruction.
Of the bone (after 4-6 weeks):
• Perform a revision
• Remove the local antibiotic carrier (Septopal)
• Irrigate intraoperatively—possibly with jet lavage
• Take smears and biopsies
• Use bone chips from the ribs (Fig. 5.56)
• Use a free fibular flap (microvascular)
• Perform fibula-pro-tibia transposition (Fig. 5.57a, b)
• Perform stable reosteosynthesis with external fixation for callus distraction:
– monosegmental transport
– bisegmental transport
– compression–distraction
Distraction is the method of first choice to bridge large bone defects in comparison to alternatives, like transplantation of rib chips, fibula-pro-tibia transposition, or microvascular bone transplantation. For a detailed discussion, see Callus Distraction, page 133 (Figs. 5.58, 5.59).

Fig. 5.56 Bone chips from ribs.

Fig. 5.57a, b Fibula-pro-tibia transposition.

Fig. 5.58a-d The monosegmental transport assembly for callus distraction requires proximal or distal corticotomy and stepwise transport of the segment in the defect.
NOTE
The daily transport distance is 1 mm in four individual steps of 0.25 mm each.

Fig. 5.59a-e The bisegmental transport assembly shortens treatment time by transporting both segments toward one another after proximal and distal corticotomy.
NOTE
Turn the screws 1 mm every day in individual steps of 0.25 mm.
Case Study
By shortening the defect, the compression-distraction assembly releases tension on the soft tissues. The shortened bone is lengthened by means of corticotomy and continuous distraction (Figs. 5.60, 5.61, 5.62, 5.63).
After 5 days begin tightening the screws: 4 × 0.25 mm daily
• Train the patient
• Write a plan for daily documentation of screw tightening and give it to the responsible person
• It takes twice as long for callus to mature after it has appeared

Fig. 5.60 A 34-year-old male with severe soft-tissue damage and a large bone defect. Chronic post-traumatic osteomyelitis. The compression-distraction assembly was attached after radical debridement of the soft tissue and bone resulted in a shortening of the tibia by 13 cm. Corticotomy

Fig. 5.61 Callus distraction to equalize the leg length by means of a Regazzoni external fixateur took 5 months.

Fig. 5.62 Regazzoni fixator. Arrows indicate the Rendel screw, which was turned 0.25 mm 4 × daily.

Fig. 5.63a, b No bone consolidation was observed at the docking site. Therefore, plate osteosynthesis was performed with autogenous bone grafting. The antibiotic chains were able to be left in place.
Bibliography
Blaha JD, Nelson CL, Frevert LF, et al. The use of septopal (polymethylmethacrylate beads with gentamicin) in the treatment of chronic osteomyelitis. Instr Course Lect 1990;39:509–514
Blaha JD, Calhoun JH, Nelson CL, et al. Comparison of the clinical efficacy and tolerance of gentamicin PMMA beads on surgical wire versus combined and systemic therapy for osteomyelitis. Clin Orthop Relat Res 1993;295(295):8–12
Bonnaire F, Hohaus T, Cyffka R, Lein T. Bone infections. [Article in German] Unfallchirurg 2002;105(8):716–731, quiz 731, 733
Burri C, Henkemeyer H, Spier W. The treatment of chronic osteomyelitis. Acta Orthop Belg 1975;41(2):235–243
Burri C. Posttraumatische Osteomyelitis. Bern: Huber; 1979
Calhoun JH, Mader JT. Antibiotic beads in the management of surgical infections. Am J Surg 1989;157(4):443–449
Calhoun JH, Henry SL, Anger DM, Cobos JA, Mader JT. The treatment of infected nonunions with gentamicin-polymethylmethacrylate antibiotic beads. Clin Orthop Relat Res 1993;295(295):23–27
Calhoun JH, Anger DM, Ledbetter BR, Cobos JA, Mader JT. The lizarov fixator and polymethylmethacrylate-antibiotic beads for the treatment of infected deformities. Clin Orthop Relat Res 1993;295(295):13–22
Cierny G III, Mader JT. Approach to adult osteomyelitis. Orthop Rev 1987;16(4):259–270
Cierny G III. Infected tibial nonunions (1981–1995). The evolution of change. Clin Orthop Relat Res 1999;360(360):97–105
Cottias P, Tomeno B, Anract P, Vinh TS, Forest M. Subacute osteomyelitis presenting as a bone tumour. A review of 21 cases. Int Orthop 1997;21(4):243–248
Dirschl DR, Almekinders LC. Osteomyelitis. Common causes and treatment recommendations. Drugs 1993;45(1):29–43
Gustilo RB, Merkow RL, Templeman D. The management of open fractures. J Bone Joint Surg Am 1990;72(2):299–304
Henry SL, Hood GA, Seligson D. Long-term implantation of gentamicin-polymethylmethacrylate antibiotic beads. Clin Orthop Relat Res 1993;295(295):47–53
Holtom PD, Smith AM. Introduction to adult posttraumatic osteomyelitis of the tibia. Clin Orthop Relat Res 1999;360(360):6–13
Khouri RK, Shaw WW. Reconstruction of the lower extremity with microvascular free flaps: a 10-year experience with 304 consecutive cases. J Trauma 1989;29(8):1086–1094
Klemm K. Gentamicin-PMMA-beads in treating bone and soft tissue infections (author’s transl). [Article in German] Zentralbl Chir 1979;104(14):934–942
Klemm K. Indication, technic and results using the external fixator in infected fractures and infected pseudarthrosis. [Article in German] Langenbecks Arch Chir 1982;358:119–124
Klemm K, Schnettler R. The use of gentamicin-PMMA chains in the treatment of infected tibial nonunion. Acta Orthop Belg 1992;58(Suppl 1):222–226
Klemm KW. Antibiotic bead chains. Clin Orthop Relat Res 1993;295(295):63–76
Klemm K. The use of antibiotic-containing bead chains in the treatment of chronic bone infections. Clin Microbiol Infect 2001;7(1):28–31
Lazzarini L, De Lalla F, Mader JT. Long Bone Osteomyelitis. Curr Infect Dis Rep 2002;4(5):439–445
Mader JT, Mohan D, Calhoun J. A practical guide to the diagnosis and management of bone and joint infections. Drugs 1997;54(2):253–264
Mader JT, Shirtliff ME, Calhoun JH. Staging and staging application in osteomyelitis. Clin Infect Dis 1997;25(6):1303–1309
Mader JT, Cripps MW, Calhoun JH. Adult posttraumatic osteomyelitis of the tibia. Clin Orthop Relat Res 1999; 360(360):14–21
Mader JT, Shirtliff ME, Bergquist SC, Calhoun J. Antimicrobial treatment of chronic osteomyelitis. Clin Orthop Relat Res 1999;360(360):47–65
Mader JT, Wang J, Calhoun JH. Antibiotic therapy for musculoskeletal infections. Instr Course Lect 2002;51:539–551
Ostermann PA, Henry SL, Seligson D. The role of local antibiotic therapy in the management of compound fractures. Clin Orthop Relat Res 1993;295(295):102–111
Ostermann PA, Seligson D, Henry SL. Local antibiotic therapy for severe open fractures. A review of 1085 consecutive cases. J Bone Joint Surg Br 1995;77(1):93–97
Perry CR, ed. Bone and Joint Infections. London: Martin Dunitz; 1996
Schnettler R, Lieser H, Klemm K. Chirurgische Behandlung der posttraumatischen chronischen Osteomyelitis. Akt Chir 1997;32:18–22
Schulte J, Burri C. Treatment results using PMMA-chains in Ulm. [Article in German] Aktuelle Probl Chir Orthop 1979; (12):158–160
Seligson D. Orthopedic infections. Orthopedics 1994;17(5):393–394
Seligson D, Ostermann PA, Henry SL, Wolley T. The management of open fractures associated with arterial injury requiring vascular repair. J Trauma 1994;37(6):938–940
Seligson D, Klemm K. Adult posttraumatic osteomyelitis of the tibial diaphysis of the tibial shaft. Clin Orthop Relat Res 1999;360(360):30–36
Tetsworth K, Cierny G III. Osteomyelitis debridement techniques. Clin Orthop Relat Res 1999;360(360):87–96
Trampuz A, Osmon DR, Hanssen AD, Steckelberg JM, Patel R. Molecular and antibiofilm approaches to prosthetic joint infection. Clin Orthop Relat Res 2003;414(414):69–88
Widmer AF. New developments in diagnosis and treatment of infection in orthopedic implants. Clin Infect Dis 2001;33(Suppl 2):S94–S106
Zumiotti AV, Teng HW, Ferreira MC. Treatment of post–traumatic tibial osteomyelitis using microsurgical flaps. J Reconstr Microsurg 2003;19(3):163–171
Local Surgical Treatment of Osteomyelitis with a Resorbable, Osteoconductive Antibiotic Carrier
R. Kraus, U. Schiefer, R. Schnettler
Introduction
The gold standard in local antibiotic therapy for the treatment of osteomyelitis is presently still the application of PMMA chains containing antibiotics. The main disadvantage of these materials is, among others, the limited choice of commercially added antibiotics (gentamicin and, with limitations, vancomycin). Individual mixing in of several other antibiotics has been described, but the mixture and respective release characteristics of the antibiotics, are uncertain; hence this procedure could involve legal problems. In addition, the chains have to be removed at a later date, and antibiotic chains can only temporarily fill in bone defects as spacers.
Collagen fleece containing an antibiotic has been suggested as an alternative, but the choice of antibiotic for this purpose is limited to gentamicin. The allogenic carrier collagen often causes incompatibility reactions with protracted wound secretion and disturbs wound healing, especially if the fleece is placed near the surface.
For the further development of local antibiotic therapy for osteomyelitis, the following characteristics of carrier materials are desirable:
• applicability of various antibiotics with defined concentrations and reliable elution characteristics
• complete resorbability to avoid the necessity of later surgical removal
• suitability as a bone replacement substance with osteoconductive characteristics to fill in or bridge the defect









