Truncus Arteriosus
Timothy C. Slesnick
John P. Kovalchin
Persistent truncus arteriosus is an uncommon congenital cardiac defect in which a single great artery arises at the base of the heart and gives origin to the coronary, pulmonary, and systemic arteries. It is present in 1% to 4% of patients with congenital heart defects, and male and female children are equally affected. Truncus arteriosus can occur as an isolated cardiac defect, in association with other cardiac malformations such as abnormal coronary artery origins and right aortic arch, or with anomalies of other organ systems, such as DiGeorge syndrome. Truncus arteriosus typically is fatal in infancy without intervention, with a mean age of death at 5 to 10 weeks and 70% to 85% mortality in the first year of life.
EMBRYOLOGY AND ANATOMY
The embryonic truncus arteriosus lies between the conus cordis and aortic sac, and at approximately week 4 to 5 of fetal development, it begins separating into the aorta and pulmonary artery. This separation is thought to occur through fusion of two spiral ridges to form the conotruncal septum between the two great arteries and is influenced by the primary looping of the ventricles. An immigration of neural crest cells into the distal ridges occurs, and this plays a role in dividing the outflow tracts into the pulmonary and aortic trunks.
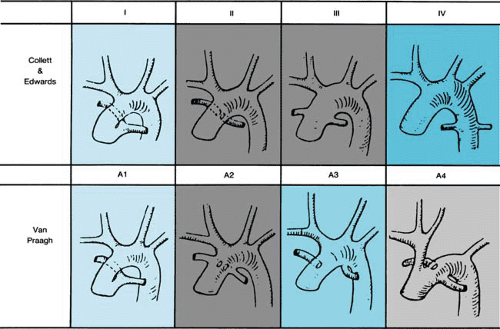
The pathologic hallmark of persistent truncus arteriosus is a single great artery arising from a single semilunar valve (truncal valve). Collett and Edwards first proposed a classification based on the anatomic origins of the pulmonary arteries. Van Praagh later proposed an alternative classification, which is more widely accepted (Fig. 263.1). In the Van Praagh classification, patients with a ventricular septal defect (VSD) are placed in group A, whereas those without a VSD (rare) comprise group B. Among both groups A and B, the four subgroups are identical. Type IV defects from Collett and Edwards’ classification now are thought to represent pulmonary atresia with VSD and sometimes are referred to as “pseudotruncus.” Similarly, the term “hemitruncus” refers to a distinct anomaly in which one pulmonary artery arises from the aorta, whereas the other arises from the right ventricle with a distinct pulmonary valve. Both these defects have a clearly different embryologic basis than that of persistent truncus arteriosus, and, therefore, both terms are misnomers.
The truncal valve has a variable number of leaflets, which often are abnormal in morphology. Approximately 65% of patients have tricuspid valves, 23% have quadricuspid valves,
9% have bicuspid valves, and occasionally unicuspid or greater than four cusps can occur. The truncal valve usually has fibrous continuity with the mitral valve, but it is rarely continuous with the tricuspid valve. Truncal valve insufficiency is present in one-half of patients with truncus arteriosus and is moderate to severe in up to one-fourth of cases. Truncal valve stenosis also has been reported in up to 30% of patients. Either regurgitation or stenosis can be caused by a variety of mechanisms, but most commonly it occurs when the valve leaflets and cusps are thickened or dysplastic. The truncal root typically straddles the VSD, with a biventricular origin in up to 80% of patients. Among the remaining patients, isolated truncal origin from the right ventricle is more common than from the left ventricle.
9% have bicuspid valves, and occasionally unicuspid or greater than four cusps can occur. The truncal valve usually has fibrous continuity with the mitral valve, but it is rarely continuous with the tricuspid valve. Truncal valve insufficiency is present in one-half of patients with truncus arteriosus and is moderate to severe in up to one-fourth of cases. Truncal valve stenosis also has been reported in up to 30% of patients. Either regurgitation or stenosis can be caused by a variety of mechanisms, but most commonly it occurs when the valve leaflets and cusps are thickened or dysplastic. The truncal root typically straddles the VSD, with a biventricular origin in up to 80% of patients. Among the remaining patients, isolated truncal origin from the right ventricle is more common than from the left ventricle.
The most commonly associated cardiac malformations with truncus arteriosus are coronary artery anomalies, occurring in one-third to one-half of patients. Aortic arch anomalies also are common findings with one-third of cases having a right arch. An interrupted aortic arch (Van Praagh type A4) is seen in 11% to 19% of patients, and conversely 12% of patients with an interrupted arch also have truncus arteriosus. Unilateral absence of a branch pulmonary artery (Van Praagh type A3) can be seen in one-sixth of patients and is ipsilateral to the side of the arch in 75% of cases.
Extracardiac anomalies also are common, seen in as many as 30% of cases, and include skeletal, renal, intestinal, and systemic defects. An association with DiGeorge syndrome has been established, such that 35% of patients with truncus arteriosus also have DiGeorge syndrome. Conversely, 9% to 11% of patients with DiGeorge syndrome have truncus arteriosus. Patients with truncus arteriosus should undergo fluorescence in situ hybridization screening for the microdeletion of chromosome 22q11, which is present in up to 90% of patients with DiGeorge syndrome.
CLINICAL MANIFESTATIONS
Most patients with truncus arteriosus present during early infancy, often in the first month of life. Fetal echocardiography allows diagnosis prenatally, although outflow tract anomalies can be difficult to visualize in the standard four-chamber view. Prenatal diagnosis allows delivery and evaluation to occur in a center experienced in surgical correction of the defect. Patients not diagnosed prenatally typically present with congestive heart failure, and symptoms depend on the volume of pulmonary blood flow and severity, if any, of truncal valve regurgitation. Infants with large volumes of pulmonary blood flow may have no visible cyanosis from birth, but the persistence of elevated pulmonary resistance in the first few weeks of life may cause mild cyanosis. As pulmonary resistance drops, however, pulmonary blood flow increases with greater mixing of saturated than of desaturated blood, and the cyanosis becomes less apparent. Tachypnea, tachycardia, difficulty with feedings, and irritability may be the first clinical signs of heart failure resulting from excessive blood flow through the pulmonary circulation. Symptoms usually occur within the first few days of life, but they may take several weeks to become apparent, secondary to delayed decrease in pulmonary vascular resistance.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree