7.18 Temperature effects on fascia The use of heat is a common tool in the treatment of muscular disorders such as stiffness or myalgia. Early reports of heat-induced relaxation in connective tissue date back more than half a century (Rigby et al. 1959). Clinical data as well as in-vitro experiments demonstrate that heat in the therapeutic range leads to a temperature-dependent myofascial relaxation (Lehmann et al. 1970; Warren et al. 1971; Muraoka et al. 2005). In this chapter, the influence of temperature on the myofascial system under physiological and pathological conditions is addressed. Perspectives of therapeutic use and the effect on resting muscle tone are given. Under physiological conditions the skeletal muscle and the fascial components interact closely. The sophisticated motor system enables humans to lift heavy weights as well as being able to perform the most fast and graceful movements, such as playing the piano. Skeletal muscle is composed of myofibers, which are formed by confluent and organized muscle cells. Contraction is initiated by calcium (Ca2+) release from internal stores (sarcoplasmic reticulum), which activates the contractile proteins. Myosin is an enzyme, which acts as an adenosin triphosphate phosphatase (ATPase) and generates force by cross-bridge cycling against the actin filaments. Muscle relaxation is mediated by Ca2+ re-absorption into the sarcoplasmic reticulum by an ATP-consuming pump (sarcoendoplasmatic reticulum Ca2+ reuptake ATPase, or SERCA). The energy is replenished by glycolysis and the respiratory chain. All these enzymatic processes are temperature dependent and obey the logarithmic Arrhenius law (Fig 7.18.1). Fig. 7.18.1 • Contraction and relaxation parameters of skeletal muscle Isolated fascia has a lower metabolic activity and contains fewer cells and mitochondria than muscle bundles. The viscoelasticity is determined by the specific composition and properties of the filaments, the chemical bonds, and other factors such as hydration. Several studies show that the viscoelastic properties of fascia are temperature dependent, although assessment is not easy, due to technical pitfalls (Lam et al. 1990). Temperature increase in fascia of up 40°C leads to reduced stiffness and more rapid elongation of the tissue, which in part can be attributed to a higher extensibility of collagen (Lehmann et al. 1970; Warren et al. 1971; Ciccone et al. 2006; Bass et al. 2007; Huang et al. 2009; personal data). In other words, there is a heat-induced fascial relaxation (Fig 7.18.2). Vice versa, passive cooling leads to an increase in stiffness (Muraoka et al. 2005). Spinal ligaments extend with temperature, as well. In sheep, this thermal expansion has been quantified to roughly 0.5 mm per lumbar segment (Hasberry & Pearcy 1986). Fig. 7.18.2 • Stretch response of fascia at different temperatures In both cases, the myofascial imbalance results in tissue remodeling. Histologically, these areas show an involution of myofibers, an increase of connective tissue, an altered composition of elastin/collagen and invasion of myofibroblasts. These cells are typically found in scar tissue and exhibit contractile properties. The forces are strong enough to adapt wounds. There are several syndromes which describe fascial contractures, e.g., frozen shoulder, Dupuytren´s disease, Peyronie’s disease, and many others (Schleip et al. 2005). Under pathological conditions the portion of connective tissue increases. The viscoelastic properties determine range of motion, myofascial (im)balance and painful contractions. Under these circumstances it is unlikely that an individual could exercise the affected limb in a manner in which muscular activity could lead to significant heat generation. The lack of the physiological benefits of temperature-induced relaxation might support the beginning of a vicious circle. Unused skeletal muscle disappears and is replaced by connective tissue, which further limits movements. Increased susceptibility of fascial injuries, in the cold, is attributed to more rigid tissue response (Bass et al. 2007). Applying heat in the therapeutic range, which means up to 40°C, may be one means by which to prevent the negative feedback loop. It has been shown that external application of heat increases the range of motion after development of a knee joint contracture (Usuba et al. 2006). In addition, the internal thermal effects generated by ultrasound may lead to an increased range of motion (Draper & Ricard 1995). Technically, there are many other ways of warming up myofascial tissue, e.g., hot bathtub, short wave diathermy or the transdermal application of pharmaceuticals, which increase regional blood flow. The contraindications to heat application include acute inflammatory diseases, skin lesions and peripheral neuropathy because of the risk of burns. Heat effects are not confined to the biomechanical properties. There is also an influence on the central nervous system and peripheral nociceptors. However, the interaction between thermal and nociceptive pathways, and the relationship to pain perception, is a complex topic, which remains controversial (Green 2004). In summary, heat in the therapeutic range leads to relaxation of many fascial contractures associated with myofascial dysfunction. In patients with low back pain, ruptures of the Fascia thoracolumbalis with prolapse of fatty tissue and muscle have been observed (Dittrich 1963; Faille 1978). Thickened fascia in patients with low back pain may be the correlate of fascial scarring (Langevin et al. 2009). Again, external heat application has been shown to be beneficial in low back pain in a Cochrane review (French et al. 2006). Warming up the ‘frozen lumbars’ may help the individual to stay active and reduce disability. The lesson we learn from the differential temperature effects on fasciae and myofibers also helps to explain passive muscle tone (Simons & Mense 1998). Warmth leads to enhanced skeletal muscle excitability, faster contraction and relaxation parameters, as well as increased force generation. On the other hand, higher temperature leads to heat relaxation and reduced myofascial stiffness in vitro. Given that there is no voluntary innervation, this effect can also be observed in vivo. Hence, the regulation of fascial stiffness plays a major role in EMG-silent resting muscle tone. Bass C.R., Planchak C.J., Salzar R.S., et al. The temperature-dependent viscoelasticity of porcine lumbar spine ligaments. Spine. 2007;32:E436–E442. Ciccone W.J., Bratton D.R., Weinstein D.M., et al. Viscoelasticity and temperature variations decrease tension and stiffness of hamstring tendon grafts following anterior cruciate ligament reconstruction. J. Bone Joint Surg. Am.. 2006;88(5):1071–1078. Dittrich R.J. Lumbodorsal fascia and related structures as factors in disability. Lancet. 1963;83:393–398. Draper D.O., Ricard M.D. Rate of temperature decay in human muscle following 3 mhz ultrasound: the stretching window revealed. J. Athl. Train.. 1995;30(4):304–307. Faille R.J. Low back pain and lumbar fat herniation. Am. J. Surg.. 1978;44(6):359–361. French S.D., Cameron M., Walker B.F., et al. A Cochrane review of superficial heat or cold for low back pain. Spine. 2006;31(9):998–1006. Green B.G. Temperature perception and nociception. J. Neurobiol.. 2004;61(1):13–29. Hasberry S., Pearcy M.J. Temperature dependence of the tensile properties of interspinous ligaments of sheep. J. Biomed. Eng.. 1986;8:62–67. Huang C., Wang V., Flatow E.L., et al. Temperature-dependent viscoelastic properties of the human supraspinatus tendon. J. Biomech.. 2009;42:546–549. Langevin H., Stevens-Tuttle D., Fox J.R., et al. Ultrasound evidence of altered lumbar connective tissue structure in human subjects with chronic low back pain. BMC Musculoskelet. Disord.. 2009;10:151. Lam T.C., Thomas C.G., Shrive N.G., et al. The effects of temperature on the viscoelastic properties of the rabbit medial collateral ligament. J. Biomech. Eng.. 1990;112(2):147–152. Lehmann J.F., Masock A.J., Warren C.G., et al. Effect of therapeutic temperatures on tendon extensibility. Arch. Phys. Med. Rehabil.. 1970;50:481–487. Muraoka T., Omuro K., Wakahara T., et al. Influence of muscle cooling on the passive mechanical properties of the human gastrocnemius muscle. Conf. Proc. IEEE Eng. Med. Biol. Soc.. 2005;1(1):19–21. Rigby B.J., Hirai N., Spikes J.D., et al. The mechanical properties of rat tail tendon. J. Gen. Physiol.. 1959;43:265–283. Schleip R., Klingler W., Lehmann-Horn F. Active fascial contractility: Fascia may be able to contract in a smooth muscle-like manner and thereby influence musculoskeletal dynamics. Med. Hypotheses. 2005;65(2):273–277. Simons D.G., Mense S. Understanding and measurement of muscle tone as related to clinical muscle pain. Pain. 1998;75:1–17. Usuba M., Miyanaga Y., Miyakawa S., et al. Effect of heat in increasing the range of knee motion after the development of a joint contracture: an experiment with an animal model. Arch. Phys. Med. Rehabil.. 2006. 87 Warren C.G.T., Lehmann J.F., Koblanski J.N. Elongation of rat tail tendon. Effect of load and temperature. Arch. Phys. Med. Rehabil.. 1971;50:465–474.
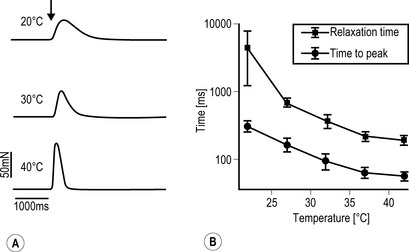
A Freshly dissected muscle strips from human m. gastrocnemius were placed in a physiological organ bath. Force registrations were performed under electrical stimulation of the tissue (0.1 Hz; 1 ms; 25 V). Twitching is clearly temperature dependent. B The contraction and relaxation parameters of skeletal muscle (human m. gastrocnemius, mean ± SEM, n = 10) obey the logarithmic kinetics of biological reactions
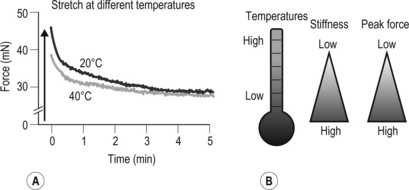
A This original force registration shows a response of 4% stretch in fascia thoracolumbalis. In a cold environment (20°C), the fascia has a higher peak force and slowed relaxation compared to 40°C. B Cartoon of temperature effects on fasciae
References
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Musculoskeletal Key
Fastest Musculoskeletal Insight Engine






