CHAPTER 12 Spine Imaging
Techniques
Plain Films
Routine plain films are universally available and inexpensive, but are limited by an inability to visualize directly neural structures and nerve root or cord compression. The presence of degenerative changes within the cervical and lumbar spine has been shown to be age related and equally present in asymptomatic and symptomatic individuals.1 By the 5th decade of life, 25% of asymptomatic patients have degenerative changes in the intervertebral disc spaces. By the 7th decade, 75% have degenerative changes. Routine spine radiographs are of little value in determining the degree and clinical severity of cervical or lumbar degenerative disc disease.
Orthogonal conventional radiography is the first line of evaluation in an instrumented postoperative patient, and plain radiographs are usually obtained at 6 weeks and 3, 6, and 12 months postoperatively.2 Regardless of which fusion approach is taken, the presence or absence of demonstrable motion or evidence of hardware failure or loosening is a key factor in the evaluation. In the case of posterolateral fusion, arthrodesis is deemed successful if follow-up radiographs show continuity in the fusion mass between the cephalad and the caudal transverse processes. Instrumented interbody fusion is considered fused if:
Pseudarthrosis or failure of fusion is indicated by progressive loss of disc height, vertebral displacement, broken or loose hardware, and loss of position of the implant or resorption of the bone graft. Flexion and extension views are useful for assessing stability or functional fusion, but the central x-ray beam should pass through the same area in both views.3
Myelography
The diagnosis of extradural neural compression by myelography is inferred indirectly by changes in the contour of normal contrast agent–filled thecal sac and root sleeves rather than by direct visualization of the lesion.4 Multiple water-soluble agents are available that provide excellent contrast and lower rates of side effects, such as iohexol (Omnipaque) and iopamidol (Isovue). Current water-soluble agents are associated with less toxicity, and their absorption through the theca and arachnoid villi makes their removal unnecessary.5 Newer nonionic water-soluble agents generally produce mild side effects, although significant adverse reactions can still rarely occur, such as hallucinations, confusion, or seizures.
The major disadvantage of myelography is its invasive nature and lack of diagnostic specificity.6 The use of less toxic second-generation, water-soluble nonionic agents has obviated the need for overnight hospitalization after the procedure. Routine postprocedural monitoring of 2 to 4 hours is usually sufficient. The technique of myelography involves instillation of the contrast agent through either lumbar puncture (midline or oblique approaches) or lateral C1-2 puncture. Adequate visualization depends on pooling of sufficient contrast agent in the region of interest to provide enough electron density to stop the x-ray beam. Absence of a significant cervical lordosis can make it difficult to concentrate the dye in the cervical region, resulting in dilution of the dye and suboptimal image quality.7 Dilution of contrast agent also occurs when attempting to visualize more than one spine region, such as lumbar and cervical. The plain film image quality of the second region studied invariably is markedly diminished (although most of these cases are diagnostically adequate by CT myelography).
Accuracy rates for water-soluble nonionic cervical myelography in the diagnosis of nerve root compression range from 67% to 92%.1,6,8,9 In a study of 53 patients with surgical confirmation of pathologic entities, myelography was associated with no false-positive findings and a 15% false-negative rate for an overall accuracy of 85%.8 Because the diagnosis of extradural neural compression is inferred indirectly by changes in the contour of the contrast agent–filled subarachnoid space, the exact nature of the compressing lesion may be uncertain. Central indentation of the dye column at the level of the disc space may be due to either compression by the disc itself or compression by a marginal osteophyte. Similarly, incomplete filling of a nerve root sleeve may be due to either a lateral disc herniation or foraminal narrowing; the distinction is sometimes difficult by myelography. There is currently almost no role for conventional myelography alone, without postmyelographic CT. The exception is the presence of stainless steel spinal implants, where CT image quality is degraded by the presence of the spinal instrumentation.
Computed Tomography
CT permits direct visualization of potential neural compressing structures and provides better visualization of lateral pathology, such as foraminal stenosis.10–12 An important benefit from a surgical perspective is the ability of CT to distinguish neural compression owing to soft tissue from compression owing to bone.9,11,13,14 CT is still limited compared with MRI in visualization of the neural structures below a complete myelographic block. What often appears as a complete myelographic block may permit passage, however, of enough contrast agent past the block to allow CT myelographic distinction.
Disadvantages of CT include radiation exposure, the effects of partial volume averaging, the time involved in performing multiple thin (1.5 to 3 mm) sections over multiple vertebral bodies and intervening discs, streak artifacts in the cervical spine caused by the dense bone of the shoulder girdle, and changes in configuration of the spine that occur between successive motion segments.15 Many of the limitations can be obviated by obtaining multiple thin sections (1.5 to 3 mm) with the gantry tilted to permit imaging parallel to the plane of the disc. Further accuracy is obtained by routinely imaging the spine by CT after the introduction of water-soluble contrast agents (intrathecal contrast medium–enhanced CT).
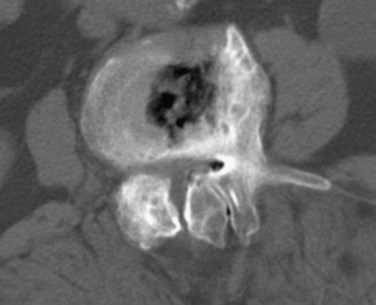
Reported accuracy rates for CT range from 72% to 91%.6,9,11,14 Agreement rates between contrast medium–enhanced CT and myelography have been reported to range from 75% to 96%.11,14 When a discrepancy exists between myelographic and CT findings, postcontrast CT is invariably the more accurate study (Fig. 12–1). New multirow detector technology is becoming available that allows for extremely rapid thin-slice acquisitions over long body segments. With this new technology, contiguous 3-mm slices can be obtained from L1 to S1 in less than 30 seconds. The acquisition of isotropic voxels allows for multiplanar reformation of the CT data with no loss in spatial resolution.
Magnetic Resonance Imaging
For most patients who present for evaluation of suspected degenerative disease, spin-echo (SE) T1-weighted and fast spin-echo (FSE) T2-weighted sagittal images and T1-weighted axial images suffice. This examination can be completed in approximately 20 minutes. If contrast between the disc and cerebrospinal fluid (CSF) is inadequate on axial images, FSE T2-weighted axial study may be useful. If there is a history of prior low back surgery, gadolinium-based intravenous contrast medium is administered, and T1-weighted sagittal and axial images are included. Patients with possible vertebral osteomyelitis can undergo this routine study. If the study shows an area that suggests a disc space infection, post–gadolinium-enhanced T1-weighted sagittal and axial sequences are often very helpful in defining disease extent and in characterizing epidural inflammatory disease.16–24
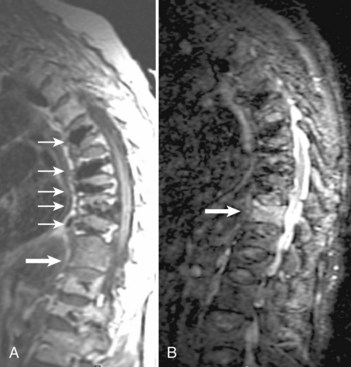
For axially oriented images, low flip angle, two-dimensional or three-dimensional, gradient-echo (GE) sequences producing “myelographic” contrast are a reasonable baseline standard of comparison, acknowledging that these sequences were developed for detecting disc herniations and are not the “gold standard” for detecting intramedullary cord lesions.17,25,26 Short tau inversion recovery (STIR) has shown a high sensitivity for musculoskeletal pathology (Fig. 12–2).27–29 STIR has been favorably compared with T1-weighted and T2-weighted FSE, conventional SE, and fat-saturated FSE in the detection of vertebral metastatic disease.30–32 STIR may also be used for intramedullary cord lesions.
For disc disease, bright CSF-type images are preferred because of the problem of visualizing low signal intensity ligaments or osteophytes against the dark CSF images on T1-weighted images.33–37 The major problem of two-dimensional MRI techniques for cervical disease is the failure to identify foraminal disease accurately owing to long echo times, relatively thick image slices (3 to 5 mm), and the inability to view the course of the exiting nerve roots in planes other than axial.38 Three-dimensional imaging allows an increase in signal-to-noise ratio over two-dimensional imaging with thin contiguous slices with a more accurate slice thickness that can be obtained without the problem of crosstalk.39,40
Artifacts
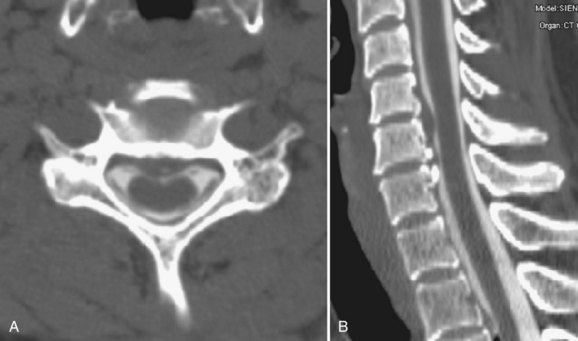
MRI studies may be severely compromised in the presence of spinal instrumentation, and there can be potential safety and biologic considerations (Fig. 12–3). There are many strategies one can employ to reduce susceptibility artifacts on MRI, including the use of SE techniques, especially FSE variants over GE; larger fields of view; higher readout bandwidths; smaller voxel sizes; and appropriate geometric orientation of the frequency-encoded direction in relationship to metallic objects. Geometric orientation is especially important in the case of pedicle screws.41 There is less apparent widening of the short axis of screws when the direction of the frequency-encoded gradient is parallel, as opposed to perpendicular to the long axis of the screw.
Spinal Angiography
Spinal angiography is extremely useful for spinal vascular malformations for the delineation of the vascular supply and for therapeutic treatment.42,43 Spinal angiography is also used in the pretherapeutic workup of suspected vascular neoplasms involving the vertebral bodies, posterior elements, and spinal canal and is coupled with preoperative or palliative embolization. Spinal angiography should address three areas for the surgeon or interventionalist: (1) the exact location and configuration of the lesion, (2) vascularity of the lesion including feeding and draining vessels, and (3) regional vascular anatomy.44
Spinal vascular malformations are a very heterogeneous group of lesions that have had a wide variety of classification schemes applied to them. One common classification system is from Anson and Spetzler,45 who classify them as types 1 to 4:
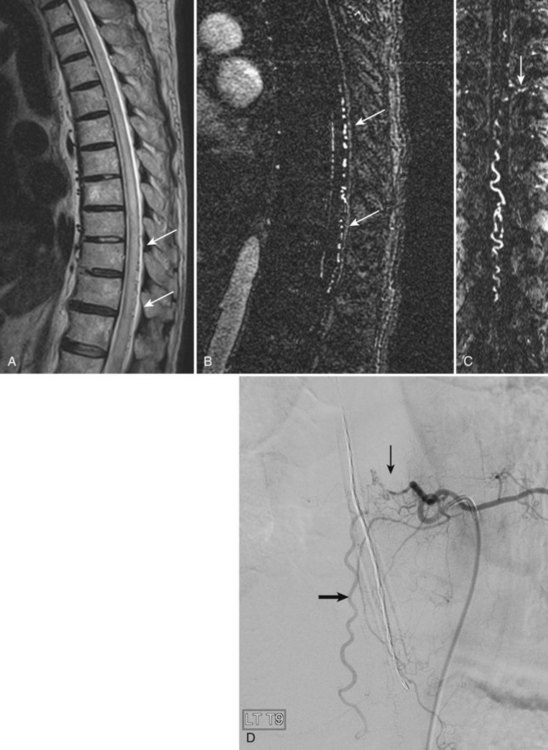
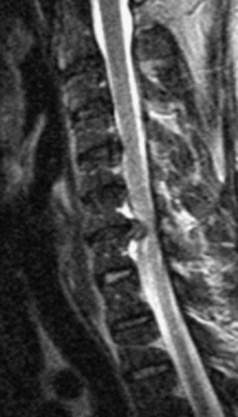
The most common spinal vascular lesion is a dural arteriovenous fistula (Fig. 12–4). These lesions are thought to be acquired and are particularly present in the thoracic and lower lumbar spine. Spinal dural arteriovenous fistulas are more common in men (3.4 : 1) older than 60 years. There is often a delay from symptom onset to time of diagnosis, averaging 27 months. Clinical findings include weakness (55%), a progressive clinical course (100%), and a myelopathy on examination (84%). The nidus of the fistula is most often located between T6 and T12 and in the sacrum and intracranially in 8% to 9% each. In 1977, Kendall and Logue46 definitively identified the site of the arteriovenous shunting within the root sleeve. The symptoms are a result of intramedullary edema and ischemia secondary to increased venous backpressure within the varicose coronal veins. Gilbertson and colleagues47,48 identified increased signal intensity on T2-weighted images within the cord as the most sensitive imaging finding in spinal dural fistula.
Although imaging, in particular MRI, has become a mainstay for the evaluation of vascular malformations, spinal angiography remains a crucial technique for precise definition of the type of lesion, the overall morphology, the flow characteristics, and the identification of specific feeding vessels.49 Selective intercostal or lumbar injection using digital subtraction angiography typically uses 2 to 4 mL of nonionic contrast agent per injection, diluted with heparinized saline. Arterial and delayed venous imaging may be necessary to appreciate fully the venous drainage of the vascular pathology, particularly in arteriovenous malformations and dural fistulas. Arterial films allow examination of abnormal blush or arteriovenous shunting. The normal vascular supply to the cord, in particular, the artery of Adamkiewicz, should be defined. In addition to the usual general complications of angiography, embolization to the anterior spinal artery could occur after angiography, which may lead to an ascending paralysis. Generally, given the small catheters used, nonionic contrast medium, and an improved speed of the examination with digital subtraction angiography, complications are rare.
Spinal Angiography Using Magnetic Resonance Imaging and Computed Tomography
Technologic advances have allowed high-resolution, high-contrast discrimination imaging for evaluation of the spinal arteries, with the goal of minimizing the need for conventional catheter angiography for identification of spinal vascular disease.50–52 The size of the anterior spinal artery (0.2 to 0.8 mm) and the close approximation of the spinal veins necessitate a sophisticated MRI sequence with bolus gadolinium–based intravenous contrast medium administration. Although various techniques may be used, the three main requirements are a large field of view, high spatial resolution, and high temporal resolution.
The large field of view should be 30 to 50 cm, which would allow visualization of the mid-thoracic and lower thoracic spine and the upper lumbar spine covering the major sources of the anterior spinal artery (70% arise from T8-L1). The high spatial resolution is required because of the small target vessel size and should employ a voxel size on the order of 1 mm or less. Temporal resolution is required to try to separate the anterior spinal artery from the adjacent vein, which is larger (0.4 to 1.5 mm). Simply trying to define the artery and vein based on morphology is extremely difficult. Temporal resolution is often in a tradeoff with spatial resolution for MRI, and time frames vary with the particular sequence and hardware, but is generally 40 to 60 seconds per acquisition to 2 to 4 minutes. Mull and colleagues52 in a series of 34 patients showed that contrast-enhanced spinal magnetic resonance angiography (MRA) could reliably detect or exclude spinal cord arteriovenous abnormalities, with a 100% predictive value. The main arterial feeder can be reliably defined by MRA, but small secondary feeders may be missed. The main reasons for obtaining MRA would be for primary identification of a vascular abnormality and to pinpoint the likely site of a feeder for conventional catheter angiography.
CT angiography can also define normal and abnormal spinal vasculature.53,54 The technique requires a multidetector row CT scanner (generally ≥16) and 1-mm section thickness. Given the tremendous speed of current CT scanners, the thoracic and upper lumbar spine can be covered in 30 seconds. As with MRA, the examination relies on a bolus of intravenous contrast medium and precise triggering of the contrast bolus at its maximum density within the thoracic aorta. Contrast injection rates are on the order of 2 to 3 mL/sec for a total of 50 to 75 mL. Considerable postprocessing is required to segment the target vessel and to connect the intraspinal vessel with the appropriate intercostal vessel to define the arterial side of the vasculature. CT angiography does not have the ability to separate out the artery and vein based on temporal resolution, as does MRA.
Discography
Discography was originally conceived as a morphologic study of disc herniation but then morphed into a useful but limited test relying on pain provocation through disc pressurization.55,56 Although discography can accurately define disc degeneration, this procedure is now seen as a physiologic evaluation of the disc consisting of volumetric, manometric, radiographic, and pain provocative challenge.57,58 This procedure remains quite controversial; it has enthusiastic supporters and detractors and has generated a voluminous literature. Some authors see discography as helpful in identifying internal disc disruption and in verifying painful disc levels before surgery (particularly fusion), whereas others see it as unproven and of questionable benefit.59–66
Discography is an invasive procedure and is not performed as a screening technique. Discography is most accurate when the diagnosis of discogenic pain is probable based on appropriate history, physical examination, and imaging.56 This test is always limited in sensitivity and specificity owing to the subjective report of pain type and location by the patient. According to Tehranzadeh and others,67–69 indications for discography include the following:
Generally, small-gauge needles (22-gauge) are placed with fluoroscopic guidance into the nucleus pulposus of one or more discs. With proper placement of the needles confirmed under fluoroscopy, contrast medium is injected into the nucleus pulposus centrally. A normal disc takes 1 to 2 mL of contrast agent. A normal disc is painless, with the contrast agent remaining centrally within the nucleus pulposus. Abnormal discs are associated with pain on injection, which simulates the patient’s symptoms and which may or may not be referred to the legs. Videotaping of the patient’s pain response and the fluoroscopic display may be performed.66 After injection of contrast agent, anteroposterior and lateral plain radiographs are obtained, followed by axial CT images through the level of the discograms. Different grading of radial pairs can be performed accurately only on the axial CT projections. The main complication for this invasive technique is a disc space infection. The main risk of discitis is 0.1% to 0.2%.70–72 A prophylactic broad-spectrum antibiotic is often used.
Magnetic Resonance Imaging Safety
The specific and important aspects of MRI safety are widely available on multiple websites, and the interested reader is referred to them for detailed answers.73–75 One more recent aspect of MRI safety that is perhaps less widely recognized outside of radiology is nephrogenic systemic fibrosis (NSF), previously called nephrogenic fibrosing dermopathy. NSF is a systemic disorder of widespread fibrosis that has been tied to prior administration of gadolinium-based contrast agents in the setting of renal disease. The incidence of NSF in the setting of severe renal dysfunction is approximately 1% to 7% after exposure to gadolinium-based contrast material. The U.S. Food and Drug Administration (FDA) has asked manufacturers to include a new boxed warning on the product labeling of all gadolinium-based contrast agents that are used to enhance the quality of MRI. The warning states that patients with severe kidney insufficiency who receive gadolinium-based agents are at risk for developing NSF, a debilitating and potentially fatal disease.74 Also, patients just before or just after liver transplantation and patients with chronic liver disease are at risk for developing NSF if they are experiencing kidney insufficiency of any severity.
The risk of a patient developing NSF may be minimized by the following steps:76–80
Degenerative Disc Disease
Multiple authors suggest that an imaging study is indicated in the evaluation of a patient with sciatica when (1) true radicular symptoms are present, (2) there is objective evidence of nerve root irritation on physical examination (i.e., positive straight-leg raise test), and (3) the patient has failed “conservative management” of 4 to 6 weeks’ duration.81–83 Earlier imaging is considered appropriate if clinical features raise concern regarding malignant or infectious causes or if neurologic findings worsen during observation. These recommendations are based on several studies of successful nonoperative treatment of sciatica.84–89 Imaging is recommended only for the remaining minority of patients with persistent signs and symptoms who are believed to be surgical candidates or in whom diagnostic uncertainty remains.
In addition to these observed changes within the degenerating disc, vertebral marrow signal abnormalities adjacent to the degenerating disc are common.90 Type I endplate change manifests as decreased marrow signal paralleling the endplates on T1-weighted images and increased signal on T2-weighted images. These changes reflect replacement of normal fatty marrow with fibrovascular marrow, which has greater water content. Type II endplate changes are slightly more common than type I changes, showing increased signal on T1-weighted images and isointense to slightly increased signal on T2-weighted images. Histologically, these changes correlate with fatty marrow replacement. These changes may be preceded by type I changes, and often these changes exist in combination at the same level or different levels. Type III endplate changes show decreased marrow signal on T1-weighted and T2-weighted images, a finding that correlates with endplate sclerosis seen radiographically.90
Fissures (tears) of the anulus fibrosus can also be visualized with MRI. They appear as small areas of increased signal on T2-weighted images and can enhance after contrast agent administration, presumably secondary to the ingrowth of granulation tissue into the fissure as a consequence of healing.91 Three types of annular fissures have been described, depending on their orientation relative to the concentric annular fibers.92 The high frequency of annular fissures seen in association with large disc bulges challenges the concept that the anulus fibrosis is intact in bulging discs but ruptured in herniated discs. The clinical significance of annular fissures is unknown. In patients without nerve root compression, back pain may be secondary to irritation of the nerve endings in the peripheral anulus either from scar tissue within an annular fissure or from a disc herniation; this is what is referred to as discogenic pain. Although this concept is often used to ascribe clinical significance to these lesions, many asymptomatic patients harbor annular fissures.
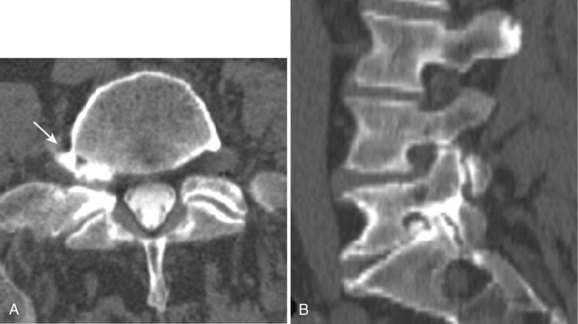
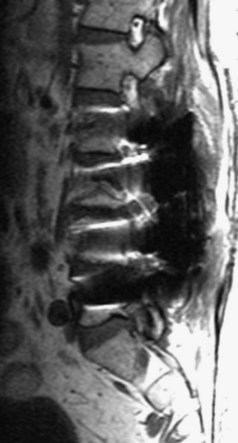
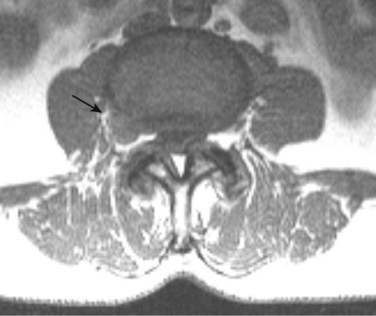
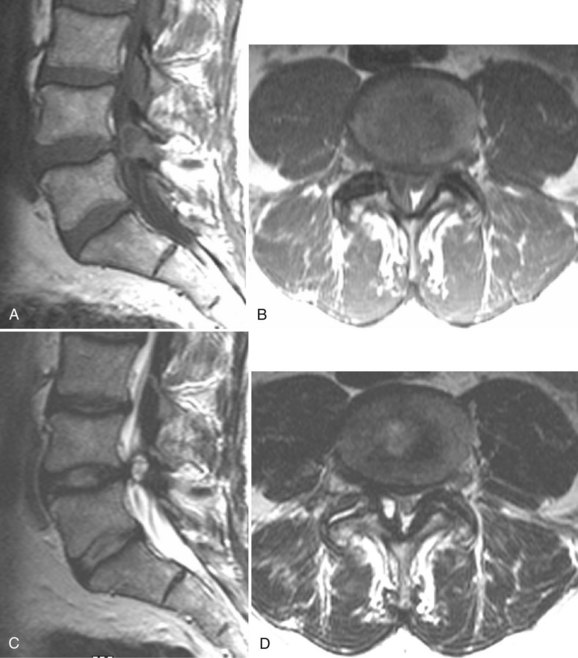
There is no universally accepted classification system describing degenerative disc disease. A multispecialty task force released recommendations for disc nomenclature spanning the orthopaedic, neurosurgical, and radiologic communities.93 This group has defined a protrusion as a herniation that maintains contact with the disc of origin by a bridge as wide as, or wider than, any diameter of the displaced material (Fig. 12–5). An extruded disc is a larger herniation where the diameter of the disc material beyond the interspace is wider than the bridge, if any, that connects it to the disc of origin (Fig. 12–6). A sequestered (free) disc fragment is an extrusion that is no longer contiguous with the parent disc. It may reside either anterior or posterior to the posterior longitudinal ligament or rarely may be intradural (Figs. 12-7 and 12-8). A free fragment may be located at the disc level or may migrate superiorly or inferiorly, often lateralized by the thin, sagittally oriented midline septum seen in the lower anterior epidural space.
Lumbar Stenosis
As an anatomic entity, spinal stenosis refers to narrowing of the central spinal canal, neural foramina, or lateral recesses. Most commonly, it is acquired secondary to degenerative disease of the intervertebral disc or facets or both, although developmentally shortened pedicles are an important component of symptomatic spinal stenosis in patients with otherwise mild degenerative changes (Figs. 12-9 and 12-10).94 Before the development of MRI, plain films and CT were used to diagnose spinal stenosis by measuring the dimensions of the bony canal. At present, such measurements are not commonly performed. These measurements do not take into account the normal anatomic variation between patients or the role of the disc and ligamentum flavum in spinal stenosis and are inaccurate predictors of clinical symptoms. MRI accurately depicts the degree and cause of thecal sac narrowing in patients with central canal stenosis. Such narrowing is most commonly due to bony and ligamentous hypertrophy.
Facet Disease
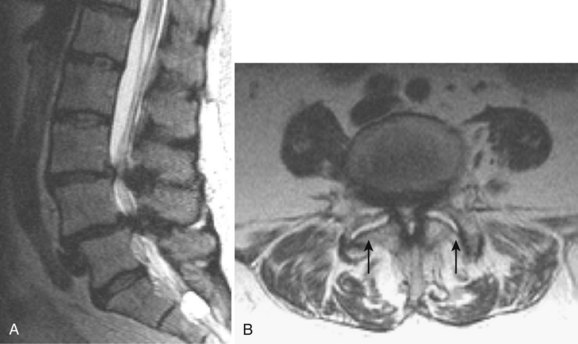
Degenerative disease of the facet joints typically occurs in combination with degenerative disc disease, although facet disease alone may be responsible for symptoms of back pain and radiculopathy. As with any synovial-lined joint, facet joints are susceptible to the development of joint space loss, subchondral sclerosis and cyst formation, osteophytosis, and subluxation. Because of the richly innervated synovium and joint capsule, these changes alone can be a source of pain, or alternatively they can contribute to nerve root impingement by causing spinal stenosis or foraminal compromise. On MRI, degenerated facets appear hypertrophied, sclerotic, and irregular. Enlarged ligamentum flavum is commonly present. Facet degeneration can lead to the formation of synovial cysts that can compress the thecal sac and roots from a posterior direction. Synovial cysts are best depicted on axial images and appear as posterolateral epidural masses adjacent to a degenerated facet, most commonly at the L4-5 level. Synovial cysts have variable signal characteristics secondary to varying cyst fluid composition and associated hemorrhage, calcification, or gas within the cyst (Fig. 12–11).95 A peripheral hypointense rim on T2-weighted images related to calcification may be seen. Intravenous contrast medium is useful in suspected cases to define better the lesion and its relationship to the adjacent facet joint and thecal sac.
Instability
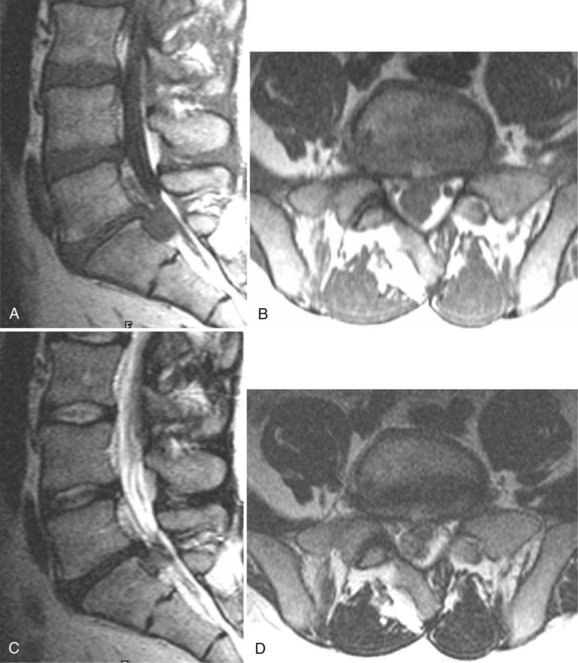
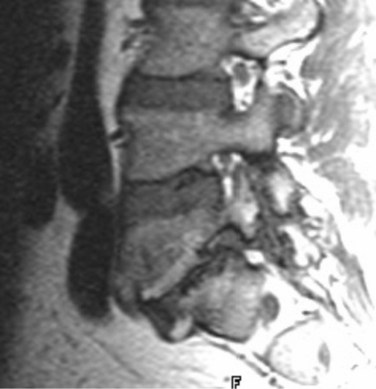
Because of its ability to obtain direct sagittal images free of overlapping structures and patient rotation, MRI is an accurate method of diagnosing spondylolisthesis. MRI is nearly always performed with the patient supine, however. In that position, a vertebra with subluxation can be normally aligned. A more accurate method of detecting listhesis is by weight-bearing lateral lumbar radiographs. The detection of spondylolysis (pars interarticularis defect without ventral slippage) by MRI can be problematic, and it is generally agreed that plain films and CT are more reliable for its diagnosis. Because MRI is being increasingly used as the first and only imaging modality in evaluating patients with low back pain and radicular symptoms, many cases of spondylolysis are imaged without the benefit of correlative plain films or CT studies.96 Using MRI, sagittal T1-weighted images are best for showing the pars interarticularis owing to their higher signal-to-noise ratio, the depiction of the pars marrow as hyperintense, and the minimal obliquity of the pars in this imaging plane (Fig. 12–12). If the pars appears normal (i.e., contiguous normal marrow signal), one can be certain that it is intact.97 The presence of abnormal pars signal is not specific for spondylolysis, however, because benign sclerosis, partial volume averaging with an adjacent degenerative facet, and osteoblastic metastases can also give this appearance.
Cervical Radiculopathy and Myelopathy
Various studies have shown that canal size is reduced in patients with cervical spondylotic myelopathy. The normal diameter of the canal from C3 to C7 is approximately 17 mm and can be decreased to 12 mm or less in cervical spondylotic myelopathy. The size that is associated with myelopathy has ranged, however, from less than 10 mm to 14 mm. Additionally, myelopathic symptoms tend to occur when the canal cross-sectional area is less than 60 mm2. The ratio of the anteroposterior canal diameter to the vertebral body diameter has been used to assess cervical stenosis. This Pavlov ratio (sometimes referred to as the Torg ratio) is normal if it is 1 or greater.98 A ratio of 0.8 or less is considered abnormal. As a ratio, however, it can be abnormal not only because of an abnormally small canal diameter (small numerator), but also because of an abnormally large vertebral body (large denominator). This ratio method also does not take into account the size of the spinal cord itself. As an isolated tool, this method is of historical interest only and is useless in evaluating cervical spinal cord compression.
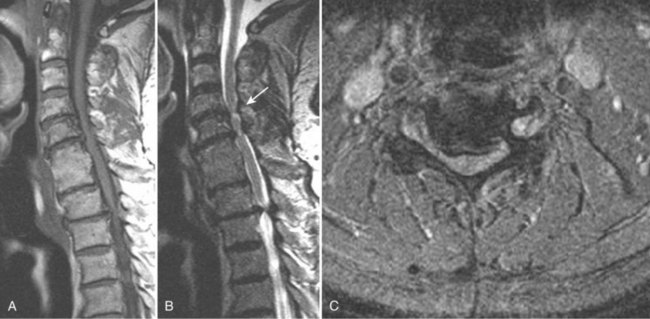
Takahashi and colleagues99 and others have described areas of increased signal intensity on T2-weighted images within the cervical cord owing to extradural compression, which variously reflects myelomalacia, gliosis, and demyelination and edema (Fig. 12–13). Patients who show areas of abnormal signal within the cord tend to have a worse clinical condition than patients with normal cord signal intensity. These abnormal signal changes can disappear or diminish after surgery to relieve the cord compression.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree